Ascorbic Acid Determination
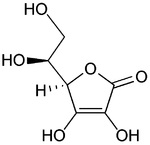
Vitamin C in food products
Many processed beverages are fortified with Vitamin C to improve the nutritional appeal of the product to the consumer. Per US government guidelines (21CFR101.9), the recommended daily intake (RDI) is 60mg/day. This recommended intake is somewhat controversial, and some would argue that much higher intakes are more beneficial for immune response. This vitamin is presented as a percentage of RDI on nutritional labeling.
A food processing plant that has a strong quality assurance program will verify the nutritional declarations on the printed label before releasing product to the public. Ideally, the vitamin content would be verified by HPLC (high performance liquid chromatography). The high cost of using this method is very unattractive, and alternative methods are often evaluated to reduce costs.
Vitamin C and Test Methods
Some popular methods for vitamin C determination include a reaction of ascorbic acid with iodine, and back-titrations with sodium thiosulfate with starch indicator. Automated methods will utilize oxidation-reduction (redox) probes to monitor the electric potential of the test sample.
Definitions:
Ascorbic acid: formula C6H8O6, is a sugar acid with antioxidant properties. Its appearance is white to light-yellow crystals or powder. It is water soluble. It is also known as Vitamin C.
Sulfuric Acid: formula H2SO4 - This acid is popular with with this titration as it easily acidifies the testing solution without reacting with the iodine or ascorbic acid.
Iodine: formula I2. The most useful form of iodine for titrations would be iodine mixed with potassium iodide (KI). Certified iodine is ideal for this kind of testing.
Starch Indicator: Starch solution is a useful aid for monitoring the reaction of iodine and ascorbic acid. When iodine is in excess - just past the equilibrium point - the starch and iodine form a complex that is purple/blue color.
Oxidation-Reduction (redox) Reaction: A chemical reaction in which the atoms involved in the reaction have their oxidation numbers changed.
Oxidation-Reduction Potential (ORP): The potential that describes whether a species wants to donate or accept electrons from other species in a redox reaction. The electrode potential is determined by the Nernst equation and is controlled by the oxidant-reductant ratio. This can be measured with a combination ORP probe.
Titrations:
Iodimetric Titration: A direct titration. It is a qualitative, volumetric procedure used in analytical chemistry to determine the concentration of an analyte (ascorbic acid) in solution.
Iodometric Titration: An indirect titration. Titration with iodine past the equilibrium point, then back-titrated with sodium thiosulfate.
Automated method: Can be either direct or indirect titration. An auto-titrator and ORP probe would need to be available to the user.
Blank - Starting point
Water, acid and starch will be colorless when starting. Ideally, a clear glass beaker, stir bar, and stir plate will be available to you.
The use of class A glassware will produce the most repeatable results. This includes all volumetric measuring and dispensing glassware.
Titrating with the iodine will produce a blue/purple solution. This is the reaction equilibrium point and it needs to be documented.
Starting point

Endpoint

Normality check of Iodine
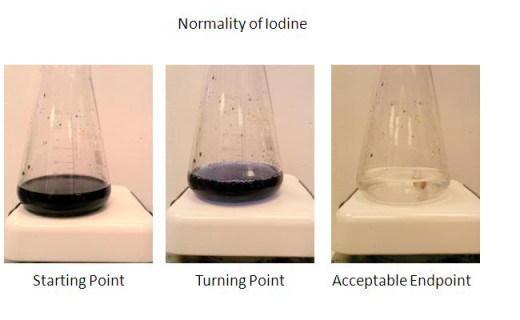
Normality check
Volumetrically measured amount of iodine in an acidified solution of water, starch and sulfuric acid can be titrated with sodium thiosulfate to verify iodine normality.
Starting point: Very blue/purple color as the iodine has formed a complex with the starch in an acidified solution.
Turning point: Slight color change is noted.
End point: From the turning point to the end point is only 1 drop of iodine. Solution turns clear. Record the volume of thiosulfate dispensed.
With the knowledge of how much iodine we started with, and how much thiosulfate was titrated we can easily calculate the normality of the iodine.
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
Example:
5mL of Iodine solution is titrated with 2.01mL of 0.1N Sodium thiosulfate. Calculate the normality of the Iodine solution. We see from the above equation that 1 mol of iodine will react with 2 mol of the thiosulfate.
Normality of Iodine (mol/L)I2 = (2.01mLthiosulfate)*(0.1mol/L)thiosulfate*(1molI2/2molthiosulfate)*(1/5mLI2)*(1L/1000mL)thiosulfate*(1000mL/1L)I2
= 0.0201 mol/L
Iodimetric Method with Iodine (manual)
Certified iodine in solution with potassium iodide is a perfect titrant for this reaction. Iodine should be stored in dark bottles and exposure to light must be avoided to prevent degradation of the iodine. The normality of iodine should be checked with sodium thiosulfate before using it. For food products, with vitamin C levels typically present in low concentrations - I'd recommend using 0.02N Iodine solution. The use of accurate glassware is important. Whenever possible you should use Class A glassware, as this will reduce the margin of error introduced by the inaccuracies of volumetric measurements. A class A 10mL burette is perfect for testing vitamin C in food products.
The testing sample is prepared by adding a measured amount of sample to distilled water and enough sulfuric acid to drop the pH below 3. Sulfuric acid should be diluted down to around 10%. Add a bit of starch as well. The well mixed sample is titrated with iodine solution, and a final titration volume is recorded. It isn't a bad idea to run a blank with just the water, starch and acid. Sometimes the color change with the iodine isn't noticed right away and this needs to be factored into reporting results.
The titration reaction:
1. Iodine solution - KI and I2 produce triiodide which is the key for this titration. Certified, stabilized iodine is best, and very reliable.
I- + I2 --> I3-
2. Reaction of iodine with ascorbic acid. It is important to note that when iodine oxidizes the ascorbic acid – 2 electrons are freed and this plays a role in the stoichiometry of the reaction for calculation purposes.
C6H8O6 + I3- + H2O ---H2SO4---> Dehydroascorbic acid + 3I- + 2H+
3. The math:
Vit C (mg/1mL) = (VI2 - blank)*NI2*Eq*MWAA*(1/vol)
Making sure we have correct units:
mgAA/1mLsample = [(mLI2-mLI2)*molI2/LI2*(1molAA)/(2molI2)*(gAA)/(molAA)*(1000mg/1g)AA)*(1L/1000mL)I2]*[1/(mLsample)]
VI2 = Volume of titrant (mL)
NI2 = Normality of Iodine (eq/L)
Eq = Equivalence ratio [from 2.]
MWAA = Molecular weight of Vitamin C (176.12g/mol)
vol = Volume of sample (mL)
blank = volume of iodine titrated to see an endpoint - on just water, acid and starch. No product.
Example:
Measuring 10mL of a food sample with a class A pipette and titrating with 0.02N Iodine solution yields a final titration volume of 5.52mL. Part 1: Taking into account a titration of a measuring blank of 0.35mL - calculate the Vitamin C in mg/mL. Part II: If a serving size is 236g, calculate the total vitamin C in 1 serving and the % of RDI.
Part I:
Vit C (mg/1g) = (VI2 - blank)*NI2*Eq*MWAA*(1/vol)
= (5.52mLI2 - 0.35mLI2)*(0.02molI2/LI2)*(1molAA)/(2molI2)*(176gAA/molAA)*(1/10mLsample)*[(1000mg/1g)AA*)*(1L/1000mL)I2]
= (5.17)*(0.02)*(1/2)*176*(1/10) mgAA/gsample
= 0.9099 mgAA/gsample
Part II:
For a 236g serving: 236gsample/serving * 0.9099mgAA/gsample = 214.7mgAA/serving.
RDI for Vit C is 60mg/serving. Therefore 214.7/60 = 357% of RDI.
Sources of error include - Error tolerances on the volumetric pipette and burette, and noting a the first color change too late (over titrating).
Iodometric Titration Method (manual)
Very similar the iodimetric method. The difference here is you put in a known amount of iodine to your sample before you do any titrating. This amount of iodine needs to be put in so the solution passes the endpoint and will be very blue/purple in color. Then you titrate with the sodium thiosulfate and record that volume until the blue/purple color goes away.
The excess iodine titrated can be determined and used to calculated the vitamin C content.
Back-titration Reaction:
I3- + 2 S2O32- --> 3I- + S4O62-
We we that the reaction stoichiometry is 1mol I3- reacts with 2mol S2O32- for our calculations.
Vit C mg/g = (I3-initial - I3-excess - blank)/(Wgt)*(MWAA)*(1/2)*(NI2)
Example:
Automated method
Really great way to do titrations. Measure your sample, and push a few buttons and the machine will monitor the potential of the solution as the reaction progresses with ORP probes and spit out an answer.
The hard part is programming the machine to recognize the endpoint, and to verify that results are acceptable as compared to known values. Once this part is done then the machine is pretty much idiot-proof.
The point in the reaction where the added iodine is stoichiometrically equal to the moles of the ascorbic acid in solution is referred to as the equivalence point, which is used to determine the amount of ascorbic acid in the test sample.
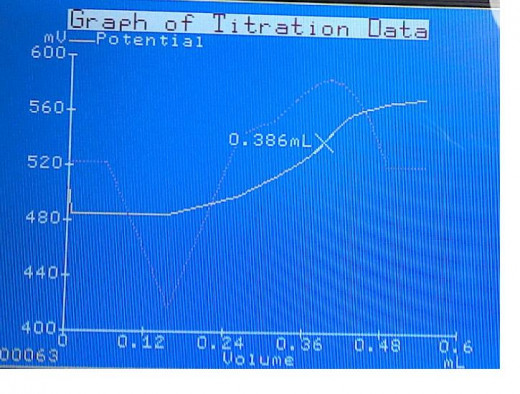
The reaction progress is monitored – A stable oxidation-reduction potential is measured (mV) by the ORP probe in the solution.
After a stable reading is determined – the unit will compare this measurement to the prior measurement(s) and will dynamically adjust (up/down) the next dose of iodine dependent on the magnitude of conductivity change.
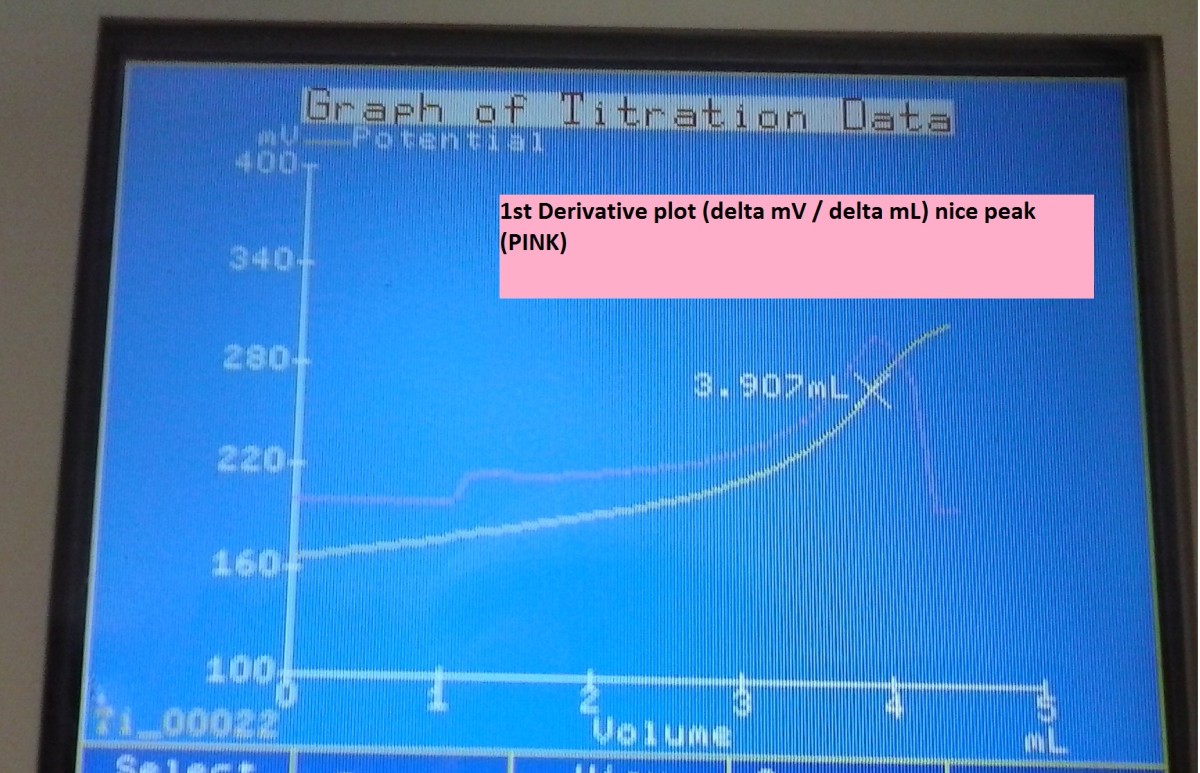
A non-linear plot of oxidation-reduction potential(mV) vs. standardized iodine(mL) is approximated by a curve that is processed in real-time. The inflection point on the curve is the titration equivalence point.
The unit calculates the 1st derivative of the approximated curve in an effort to determine the inflection point. The maximum ΔmV of the 1st derivative curve is the titration equivalence point.
Calculations can be programmed into the unit for either iodimetric method or iodometric methods as outlined above.
Automated method graph generation