Lucid Knowledge Regarding: Atomic Mass, Molecular Mass And Molar Mass.
Meaning of the term: “Atomic Mass”
The mass of single atom of any element is called atomic mass of that element.
For example, the mass of single atom of hydrogen is called atomic mass of hydrogen.
Likewise the mass of single atom of sodium is called atomic mass of sodium.
Difficulty in Determination of, “Atomic Mass”
As we know, the atom is infinitesimally small entity. It is so small that, it can not be seen or isolated by any technique. It is therefore not only difficult, but impossible to determine the mass of single atom just by weighing it in a balance.
It is also impossible to determine the mass of a sample containing known number of atoms and then to divide it by number of atoms, because there is no device to know the exact number of infinitesimally small atoms in a given sample.
It is Avogadro’s Hypothesis which solved this Problem.
The statement of Avogadro’s hypothesis is as follows.
“Equal volumes of any two gases kept under similar condition, always contain equal number of molecules”.
A deliberate study of Avogadro’s hypothesis provided an important guideline to solve the above problem.
To understand this, let us consider atomic masses of oxygen and that of hydrogen.
As per Avogadro’s hypothesis, equal volumes of Oxygen and Hydrogen when taken in two different containers (at similar condition of temperature and pressure), then the ratio of: “mass of sample of oxygen to that of hydrogen”, is actually the ratio of: “molecular mass of oxygen to the molecular mass of hydrogen”!
Experimentally, this ratio is always found to be “16:1”. This indicates that, “a molecule of oxygen is 16 times heavier than a molecule of hydrogen”.
Knowing that, the atomicity of both gases is two, it can be easily concluded that atom of oxygen is also 16 times heavier than that of hydrogen.
Thus, Avogadro’s hypothesis provided a measure to compare the atomic masses of two different elements.
It must be noted here that this does not help to determine the absolute mass of any atom. It can just help to determine the ratio of masses of two atoms.
What is meant by, “Atomicity of a Gas”?
It is the number of atoms, a given molecule of gas contains.
For example, the molecule of ozone (O3) contains three atoms of oxygen hence atomicity of ozone is taken as three.
Atomicity of some common gases is given in the following table.
Atomicity of Some Common Gases
Name of gas (its symbol)
| Atomicity
| |
|---|---|---|
Helium (He)
| 1
| |
Neon (Ne)
| 1
| |
Argon (Ar)
| 1
| |
Nitrogen (N2)
| 2
| |
Hydrogen (H2)
| 2
| |
Oxygen (O2)
| 2
| |
Chlorine (Cl2)
| 2
| |
Sulphur dioxide (SO2)
| 3
| |
Carbon Dioxide(CO2)
| 3
| |
Hydrogen sulphide (H2S)
| 3
| |
Water vapour (H2O)
| 3
| |
Sulphur trioxide (SO3)
| 4
| |
Methane(CH4)
| 5
| |
Ethane(C2H6)
| 8
| |
Propane(C3H8)
| 11
| |
Methyl isocyanate(MIC) gas-(CH3NCO)
| 7
|
The Concept of “Relative Atomic Mass”
From the above discussion it can be easily understood that, even though the actual mass of an atom can not be determined, its mass relative to mass of other atom can be determined.
Such mass of an atom is called, “Relative Atomic Mass” and is abbreviated as: r. a. m.
Here, the atom with which its mass is compared is known as, “reference atom”.
Initially, being the lightest atom, hydrogen was selected as a reference atom and its mass was arbitrarily assigned to be 1 unit. By this method, the relative mass of oxygen comes to be 16 units (to be exact, 15.88 units).
The difficulty in this method was that the relative atomic masses of most of atoms were found to be in fraction.
To avoid this, an atom of oxygen was then selected as a reference atom and its mass was arbitrarily taken to be 16 units. This also could not help much.
Nowadays, it is the atom of carbon-12(12C), which is accepted as a reference atom with assumed mass of 12 units. Based on this, the relative mass of hydrogen comes to be 1.008.
The Concept of, “Atomic Mass Unit (a. m. u.)” also known as, “Unified Mass (u)”
As the relative mass of an atom is actually a ratio of its own mass to the mass of other atom, it has no unit.
However, by convention, relative atomic masses are expressed in terms of, “atomic mass unit-a. m. u.” which is also known as, “unified mass-u.”
For this, the relative atomic mass of carbon-12 isotope is accepted to be, “12 a. m. u.”
Based on this, 1 a. m. u. can be defined as: “one twelfth mass of single atom of carbon-12 isotope”.
As per this consideration, atomic mass of hydrogen comes to be, “1.008 a. m. u.”
Identify the Lightest and the Heaviest Atom
view quiz statistics“Mass Spectrometer” is a Revolutionary Technique to Determine the Actual Mass of Single Atom
Now, science has developed a new device which can determine the mass of an infinitesimally small particle like an atom, and that too with reasonable accuracy!
The name of this advanced scientific instrument is, “Mass Spectrometer”.
The mass of carbon-12 atom as determined by Mass Spectrometer is found to be, 1.992648 x 10-23 grams.
This means 12 a. m. u. = 1.992648x10-23 grams.
By simple calculation, it can be shown that, 1 a. m. u. = 1.66x10-24 grams.
The quantity: 1.66 x 10-24 grams is known as, "gram equivalent of a. m. u."
Concept of, “Average Atomic Mass”
The atomic mass of chlorine is assigned to be 35.5 a. m. u.
Does this mean that mass of single atom of chlorine is 35.5 a. m. u.?
No, not at all!
None of the chlorine atom existing in nature has the mass of 35.5 a. m. u.!
In fact, there are two isotopes of chlorine: one with a mass of 35 a. m. u., while another with a mass of 37 a. m. u. As the natural abundance of chlorine-35 is 75% and that of chlorine-37 is 25%, the average of both is taken to be 35.5% as per calculation shown below.
Suppose, 100 chlorine atoms are taken for this purpose.
Now, among these 100 atoms; 75 atoms will have the mass of 35 a. m. u. each, while the rest of 25 atoms will have the mass of 37 a. m. u. each.
The total mass of 100 chlorine atoms will be: [(75X35) + (25X37)] = 2625+925 = 3550 a. m. u.
Hence, the average mass of one chlorine atom = 3550/100 = 35.5 a. m. u.
It is due to this reason that atomic masses of some elements are expressed in fraction.
For example, atomic mass of Neon =20.179 u, that of Argon=39.948 u, that of Carbon=12.011 u. and that of Calcium=40.08 u.
Meaning of the term: “Molecular Mass”
The mass of single molecule of any pure substance is called molecular mass of that substance. For example, the mass of single molecule of sulphuric acid is called molecular mass of sulphuric acid. Likewise the mass of single molecule of phosphorus is called molecular mass of phosphorus.
However, a molecule is formed when two or more atoms of either same or different elements combine chemically.
H2, Cl2, P4, S8 etc. are the molecules of elements; while H2O, HCl, CH4, H2SO4 are the molecules of compounds.
As molecule is made up of several atoms, molecular mass can be easily computed by summing the atomic masses of constituent atoms.
For example, molecular mass of HCl=atomic mass of 1 hydrogen atom + atomic mass of 1 chlorine atom=1+35.5=36.5 a. m. u.
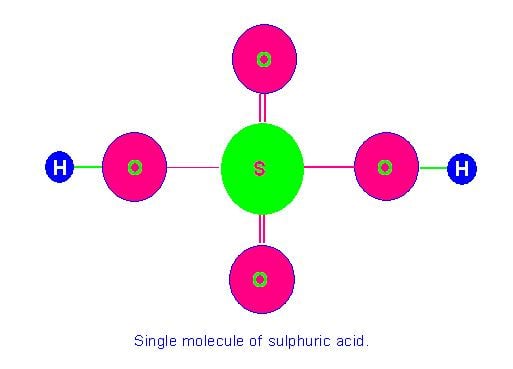
Likewise molecular mass of sulphuric acid=atomic mass of 2 hydrogen atoms + atomic mass of 1 sulphur atom + atomic mass of 4 oxygen atoms=2+32+64=98 a. m. u.
Meaning of the term: “Molar Mass”
Molar mass is defined as, “mass of 1 mole of substance”. For example, mass of 1 mole of sodium is called molar mass of sodium, and mass of 1 mole of oxygen is called molar mass of oxygen.
From the definition, it is clear that in order to understand molar mass, it is necessary to understand the meaning of the term, “mole” first (which is also called, “Mole Concept”.). Mole is the S.I. unit of quantity. In scientific terminology, the quantity of any substance is not described in terms of its mass or its volume, but is described in terms of its mole content.
‘Mole’ is defined as, “quantity of any substance that contains same number of elementary particles as there are atoms in 12 grams of carbon-12”. This means, exactly 12 gram of pure carbon with isotopic mass 12 is taken first, and then the number of atoms present in it is determined accurately. If this number is found to be, “X”, then any other substance containing “X” particles in it, is called, "1 mole quantity of that substance".
Experimentally it is found that the sample of 12 grams of carbon-12 contains 6.022x1023 carbon atoms. The number 6.022x1023 is called, "Avogadro number".
Molecular and Molar masses of some common substances
Name of substance (its symbol)
| Its molecular mass (u)
| Its molar mass (grams)
|
|---|---|---|
Calcium (Ca)
| 40 u
| 40 grams
|
Sodium chloride (NaCl)
| 58.5 u
| 58.5 grams
|
Potassium hydroxide (KOH)
| 56 u
| 56 grams
|
Picture Showing Single Molecule of Sulphuric Acid (molecular mass = 98 a.m.u.)
Picture Showing Quantity of One Mole of Sulphuric Acid (molar mass = 98 grams)

Determination of, “Avogadro’s Number"
In order to determine the exact number of atoms present in 12 gram of carbon-12 (which is known as, Avogadro's number), following calculation is carried out.
Mass of 1 atom of carbon-12 isotope=1.992648x10-23 grams.
But, mass of sample of carbon-12=12 grams.
Hence, number of atoms in 12 grams of carbon-12= 12 / (1.992648x10-23)
=6.022x1023
=Avogadro number.
Thus:
(a) Mass of 6.022x1023 atoms of calcium=molar mass of Ca=20g/mol.
(b) Mass of 6.022x1023 atoms of sodium=molar mass of Na=23g/mol.
(c) Mass of 6.022x1023 atoms of hydrogen=molar mass of H-atom=1g/mol.
(d) Mass of 6.022x1023 molecules of sulphuric Acid=molar mass of H2SO4=98g/mol.
(e) Mass of 6.022x1023 molecules of water=molar mass of H2O=18g/mol.
This means, if the constituent particles of given substance are atoms (like Na, Ca etc.), then its molar mass is equal to its atomic mass expressed in g/mol. However, if the constituent particles of given substance are molecules (like HCl, H2O etc.), then its molar mass is equal to its molecular mass expressed in g/mol.
Previous
- Lucid Science Of Evaporation Accompanied With Cooling
Why We Feel Cold While Standing In Open Atmosphere With Wet Body? Why Tea In Soccer Gets Cooled Early Compared To That In A Cup? Why Soup In Bowl Gets Cooled Early While Stirring It Continuously?
Next
- Lucid Understanding Of, “Dipole Moment Of A Molecule”:
Do You Know On Which Basis Shape Of A Molecule Is Determined? Do You Know How Percentage Of Ionic Character Of A Covalent Compound Is Determined?