Lucid Science Of Evaporation Accompanied With Cooling
What is Evaporation?
Evaporation is a spontaneous (means natural) phenomenon which converts the volatile liquid into its gaseous state, at any condition of temperature and pressure prevailing in the surrounding.
Thus the phenomenon of evaporation does not require any specific condition of temperature and / or pressure.
This means, evaporation can take place:
(a) In hot weather like summer (remember ponds and lakes become dry during summer)
(b) In very cold weather like winter (remember vapors coming from ice) and also
(c) In humid weather like monsoon (remember cloths get dried in monsoon).
This means the phenomenon of evaporation can not be stopped but can be minimized.
Cause of Evaporation
Why evaporation takes place spontaneously? The driving force of the phenomenon of evaporation is the universal tendency of any system to attain the minimum energy level.
Which state ensures the minimum energy?
Of course it is, "The State Of Equilibrium!
When the surrounding atmosphere has less moisture than system under consideration, then moisture will pass automatically from system to surrounding to attain the state of equilibrium.
This means, in case there is no difference in the moisture content of system and surrounding, the phenomenon of evaporation will not occur at all!
Can Every Liquid Evaporate?
Of course not, only such liquids can undergo evaporation, molecules of which are joined with moderate intermolecular attractive forces. Such liquids have moderate boiling points.
The examples of such liquids are:
(a) Water (boiling point 1000C),
(b) Ethanol (boiling point 780C),
(c) Diethyl ether (boiling point 350C),
(d) Chloroform (boiling point 620C) etc.
However, liquids having high boiling points like: glycerine (boiling point 2900C), mercury (boiling point 3560C), lubricant oil, sulphuric acid (boiling point 3170C) etc. can not undergo evaporation.
Why only certain liquids undergo evaporation?
The reason for this is: energy required to break intermolecular bonds existing among the molecules of some liquids is so high that surrounding can not provide it.
[Note: Fumes coming out from concentrated sulphuric acid is not evaporation of sulphuric acid, but it is decomposition of sulphuric acid into water and sulphur trioxide gas. This can be shown by following equation.
H2SO4 (l) → H2O (l) + SO3 (g)]
However, in our day to day life, water is the most common example to consider for evaporation. This is because it exists in plenty and also as its evaporation affects us in many ways. Therefore, in general discussion, the phenomenon of evaporation is considered as evaporation of water.
What is called, "Volatile Liquid"?
The liquid which evaporates readily is called, "volatile liquid". Boiling points of such liquids are generally low. Examples of such liquids are: petrol, diethyl ether, ethanol etc.
On the contrary the liquid which does not evaporate easily is called, "non-volatile liquid". Boiling points of such liquids are generally high. Examples of such liquids are: glycerine (which is also known as "glycerol" or "Propane-1, 2, 3-triol), lubricant oil, mercury etc.
Some Common Examples of Evaporation of Water
(a) When some water is spilt on a floor, it automatically disappears after sometime.
(b) If petrol is spilt, it will disappear immediately.
(c) Before injecting a medicine into our body, when doctor applies spirit on the skin, it readily disappears from our skin.
(d) In summer, the water in reservoir evaporates leaving behind dry clot of soil.
(e) In dry season (particularly in winter), our skin loses moisture and becomes dry so that we need to apply some moisturizing cream/lotion.
(f) The moisture accumulated on our skin due to sweating in summer, disappears as soon as we stand under shadow.
(g) The formation of clouds in summer is the result of huge evaporation of water occurring from rivers and oceans.
Characteristics of “Liquid State”
Liquid state is such a state of matter in which:
(a) Constituent particles / molecules are joined with each other through a weak force of attraction. Such weak force of attraction may be either “van der Waals' force of attraction” or “hydrogen bond”. Such force is weaker compared to that present among the molecules which are in solid state, but it is stronger than that present among the molecules which are in gaseous state.
(b) Due to such intermediate (moderate) force of attraction, molecules of liquid can move about freely. Such random movement of liquid molecules is termed as, “Brownian Movement”. This means each and every molecule of liquid possesses some “kinetic energy”.
(c) The magnitude of kinetic energy of liquid molecules depends upon the temperature of liquid. More is the temperature more will be the kinetic energy.
(d) During their random movement, liquid particles continuously collide with each other. Hence all the molecules of liquid do not possess identical kinetic energy. Due to this reason, the “average kinetic energy of liquid molecules” is taken as a measure, instead of taking kinetic energy of individual molecule. This means, at a particular temperature, the average kinetic energy of all molecules remains constant, though some molecules are moving faster (possessing higher kinetic energy) while some are moving slowly (possessing lower kinetic energy).
(e) At any given temperature, there exists a fraction of molecules in the liquid having sufficiently high kinetic energy to escape from the surface of liquid into the open atmosphere (as a gas) above it.
This is the phenomenon known as, “evaporation”.
Characteristics of “Gaseous State”
The main characteristics of gaseous state are as follows.
(a) Unlike liquid, the particles of gas are not joined with each other through weak force. This means no appreciable intermolecular attraction exists among the particles of gas. Due to this, they are far apart from each other and always moving with tremendous speed. It is due to this reason that this state of matter has the highest energy among all the three states of matter.
(b) Due to absence of intermolecular attractive forces, gases possess a unique property called, “diffusion”.
(c) Gases do not have a fixed volume. They acquire the volume of the container.
(d) Gases are highly compressible.
(e) Temperature significantly affects the volume of gases.
What is the Requirement to Convert Liquid state into Gaseous State?
By now, we know that the molecules of liquid are held together by weak attractive forces. Therefore the main requirement for evaporation to take place is to break such intermolecular forces of attraction.
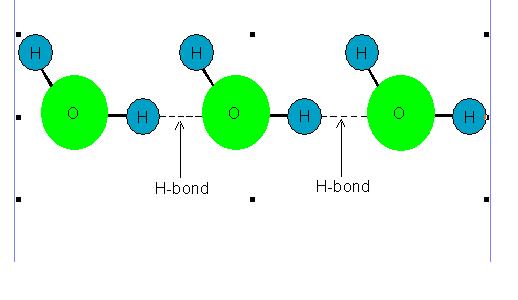
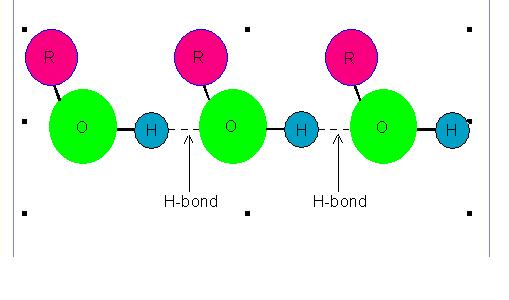
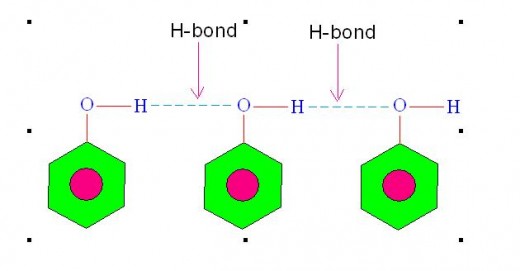
Molecules of some liquids like petrol, ether etc. are joined with van der Waals' forces hence their evaporation involves breaking of van der waals' bonds. However, molecules of some other liquids like water, ethanol etc. are joined with hydrogen bonds hence their evaporation involves breaking of hydrogen bonds.
Following picture shows hydrogen bonding existing among the molecules of some liquids.
Picture showing Hydrogen Bonding among the Molecules of some liquids
Evaporation, an Endothermic Process
Now it is clear that bonds are broken during evaporation. But breaking of bonds requires energy (in the form of heat). Hence evaporation is an, "endothermic reaction".
This means “heat is always absorbed during the process of evaporation!”
Is it possible that conversion of a liquid into its gaseous state can take place even if heat is not gained by it?
The answer is: “no, it is not possible at all”.
Undoubtedly and unexceptionally evaporation is an endothermic process.
Mechanism of Evaporation
Particles of liquid are facing two opposite forces simultaneously:
(a) Due to random motion (which is called, "Brownian motion") of constituent particles of liquid, it possesses some kinetic energy. Due to this, some particles of liquid are capable to escape into the open atmosphere from the surface of liquid.
(b) Intermolecular attractive forces hold molecules of liquid together. This does not allow the particles of liquid to escape in the atmosphere.
Under the influence of above two forces, only such particles of liquid can escape kinetic energy of which is greater than energy of attraction holding them together.
It is important to note that molecules at the surface of liquid possess higher kinetic energy compared to molecules inside the liquid. This is because the molecules at surface are directly exposed to atmosphere hence can absorb heat from the surrounding. The heat absorbed thus increases their kinetic energy. Due to this the particles of liquid at surface can easily escape from it.
Why Evaporation Brings About Coolness?
As the process of breaking of bonds is continuously taking place during evaporation, energy is continuously absorbed (in the form of heat) from the surrounding. This results in fall of temperature of surrounding.
Relation between rate of evaporation of a liquid and amount of cooling:
Rate of evaporation of a liquid and amount of cooling it produces is related with its boiling point. It is found that, liquid with higher boiling point brings about more coolness but the rate of its evaporation is slow. On the other hand, liquid with low boiling point brings about less cooling but the rate of its evaporation is high.
Why evaporation of some liquids produce more cooling?
The amount of cooling produced during evaporation depends upon molar heat of vaporization (ΔHvap) of the liquid. Higher is its value more is the amount of cooling.
Values of “ΔHvap” for some common liquids are given in the table below.
Approximate Values of Molar Enthalpies of Vaporization of Some Common Liquids expressed in kilo Joule per mol (kJ/mol) units.
Name of liquid
| Molar enthalpy of vaporization (kJ / mol)
| Boiling point (degree centigrade)
|
|---|---|---|
Water
| 40.8
| 100
|
Carbon tetrachloride
| 30.5
| 77
|
Benzene
| 30.8
| 80
|
The values of enthalpy of vaporization show that higher is the enthalpy of vaporization, stronger are intermolecular attractive forces, higher is the boiling point, lesser is the tendency of evaporation.
Each Phenomenon Leading to Change of Liquid into Its Vapor is not Evaporation.
There are three different phenomena through which a given liquid can be converted into its vapor state. These are:
(a) Evaporation,
(b) Vaporization and
(c) Boiling.
However, all these three phenomena are not covered under the definition of evaporation.
It is therefore necessary to understand the difference among them.
Table below shows important points of differences among them.
Difference among Evaporation, Vaporization and Boiling
Evaporation
| Vaporization
| Boiling
|
|---|---|---|
(1) It takes place at a temperature of surrounding.
| It takes place at any temperature.
| It takes place only at a fixed temperature, which is called boiling point of liquid.
|
(2) It is spontaneous.
| It is non-spontaneous.
| It is non-spontaneous.
|
(3) It occurs at very slow speed.
| It occurs at moderate speed.
| It occurs at very fast speed.
|
(4) It makes the surrounding cool.
| It does not make the surrounding cool.
| It does not make the surrounding cool.
|
(5) Temperature of a liquid decreases during evaporation.
| Temperature of a liquid increases during vaporization.
| Temperature of liquid remains constant during boiling.
|
(6) For example: loss of sweat from skin.
| For example: heating the water in a pan below its boiling point.
| For example: boiling of water in a pan.
|
Heating is required for vaporization and boiling, while evaporation occurs at it's own.
High Humidity Decreases the Rate of Evaporation.


Factors Affecting the Rate of Evaporation
Following factors influence the rate of evaporation.
(a) Surface area of liquid; more is the surface area of liquid, higher is the rate of evaporation. For example, tea in a saucer gets cooled faster than in a cup.
(b) Temperature of surrounding; more is the temperature of surrounding, higher is the rate of evaporation. For example, in summer water gets evaporated sooner than it does in winter.
(c) Velocity of wind; more is the velocity of wind, higher is the rate of evaporation. For example, wet floor of the room becomes dry sooner if ceiling fan is running over it.
(d) Humidity (means moisture content) of the surrounding; more is the humidity of the surrounding less is the rate of evaporation. For example, wet clothes take more time to become dry in monsoon.
Some Applications of Evaporation
(a) The evaporation of huge quantity of water from oceans and reservoirs results in "Rain" which is vital for living kingdom.
(b) "Desert Cooler"-a device of speedy evaporation, is very much useful for inside cooling of offices and homes in the summer.
(c) Evaporation of sweat from our skin helps us to maintain the temperature of our body in summer.
Condensation: the Opposite Phenomenon of Evaporation
What will happen in case moisture content of system is lesser than that present in the surrounding?
Essentially, there will be a reverse phenomenon in which moisture from surrounding will enter into system so that system can attain the state of equilibrium!
This opposite phenomenon of evaporation is called, "Condensation".
For example, droplets of dew are formed on the petals of flowers in the early morning!
Previous
- Lucid Concept Of Partition Law(Distribution Law)
When a solid is shaken with a mixture of two immiscible solvents, it gets dissolved in both the solvents but in different proportion. It distributes itself in both solvents, obeying a particular law!
Next
- Lucid Knowledge Regarding: Atomic Mass, Molecular Mass And Molar Mass.
Do You Know That The Atom Of Carbon-12 Isotope Has Two Types Of Masses? Do You Know That There Does Not Exist Any Chlorine Atom Having Mass Of “35.5 u”?