Six Hot Science Facts

What Gasses Surround Fire
A lot of people think dumping a bucket of water in a fire smothers the fire by removing the oxygen. This is somewhat correct. There are other forces at work that you never hear about. Cellulose is the main substance in wood that burns. It ignites somewhere in the range of 450 degrees fahrenheit. Read the book Fahrenheit 451? That's where Ray Bradbury got the name of the book.
What happens when you dump a bucket of water on the fire is that it removes the oxygen for only a moment. A larger force at play is that it removes the heat in the fire. It's still hot to touch, but dumping water on a fire cools it to below 450 and then the fire dies out. The wood is dry shortly after you dump the water on it, but the wood is no longer burning.
Other forces play a major role in larger fires. For example, when a house is on fire and you squirt a firehose through a window, the water inside will vaporize rapidly, both sucking the heat out of the fire as well as expanding a ball of vaporized water that pushes the oxygen out of the room and away from the fire. This can cause a miniature backdraft if the air inside is already depleted of oxygen and superheated. It does this because the air is full of superheated unburned hydrocarbons that have no air and are suddenly pushed out of the room by the expanding vapor into an area where there is oxygen to ignite the hydrocarbons. Spraying water through one window can turn another window into a momentary rocket.
Another concept most people don't understand about water... It takes a little over 4 joules of energy to heat up one gram of water by one degree celsius. It takes more than 55 times that amount of energy to vaporize one gram of water. Boiling water is at 100 degrees celsius and the steam above it is at 100 degrees celsius. Yet the steam has 200+ joules of energy per gram more than the water below it. That's one reason why steam burns are so much more damaging than getting boiling water on you. That's another reason why vaporizing the water in a fire is 55 times more effective at sucking heat out than by just heating up the water.
You ever wonder why mist machines at theme parks are so cool on hot days? That's because it's vaporizing water without the heat to do it - so the mist sucks the heat out of the air to balance the energy.

Can't Touch This
Liquid nitrogen is fun stuff to mess around with. I didn't see any up close until my third year of college. My professor dumped two or three cups onto the floor when he was done with it simply because he was done with it. At first I was thinking it would create a puddle on the floor that would soak everything - the inconsiderate jackass. But no, it actually burst into hundreds of little liquid droplets that skittered across the floor collecting dust as they went. They eventually moved downhill, showing you the low spots on the floor that were too shallow to perceive with your eyes or feet. Within thirty seconds they had all evaporated, leaving little clusters of dust and a group of students staring at it with dumb looks on their faces.
A year later I watched a dude quickly stick his finger in a vat of liquid nitrogen and quickly pull it out. He was untouched! I was expecting to see his finger turn into a brick of ice like you see in Hollywood - you know the terminator freezes and shatters... stuff like that. So this guy does this trick with liquid nitrogen over and over, rolling some around in his hands and even some in his mouth. I was stupified!
My teacher came to explain to my ignorant mind... The heat of your body is so hot relative to the liquid nitrogen that it vaporizes before it actually touches your skin. This early evaporation means that it will create a pocket of vaporized nitrogen around your finger that will insulate your finger from the tremendous cold. Now that vapor is still really cold so it would be wise not to keep your finger stuck in the nitrogen or to let the nitrogen settle in one spot in your hand or mouth. You got to keep it moving. If you don't, you will get freezer burn on the spot where the nitrogen is.
Vaporized nitrogen will still suck heat out of your skin, but the cold takes a second to bite. Nitrogen heats up quickly so when your skin is near it, the damage caused by the cold vapor is minimal. What really sucks is when you bump into something that doesn't heat up that quickly that has been in contact with liquid nitrogen - your clothes if you've spilled nitrogen for example. The nitrogen will freeze your clothes and you won't know it until you shift position and the frozen cloth touches your skin - instant freezer burn (aka frostbite). If you put small equipment in liquid nitrogen and let it cool down to super cold - that equipment you can touch and it will suck heat out of your skin tremendously quick. We would dip things in liquid nitrogen with tongs and then accidentally grab the tongs on the wrong end - burning off parts of our fingerprints in the process. That really blows. Trust me.
Not to admit horseplay or anything, but once I dipped a small vial in liquid nitrogen and let it cool down. I partially filled the vial with nitrogen and capped it. I knew the nitrogen would vaporize and expand, building pressure inside the vial until it would pop - a miniature liquid nitrogen bomb. I was expecting a small pop like when you pull your Garfield doll off a window and the suction cups make that little snap sound. I imagined that the lid would fly maybe six inches in the air and roll around when it landed. I thought this would happen quickly. No. Ten minutes later, when I was bored of watching it, the vial blows off the damn desk, the center part of the lid blew so far away so fast none of us ever saw it again. The edge of the lid was frozen tight to the vial, which we found under the fridge. People came bursting into the room to find out what the hell exploded. We all claimed ignorance. Tip for you: don't make liquid nitrogen bombs - it will throw liquid nitrogen and shrapnel in unexpected places. We were lucky no one got hurt.
It was still really fun though. We would drop flies in the liquid nitrogen, shatter frozen racquetballs, shatter frozen latex gloves... Every scientist has a hooligan living inside him.
Oxygen Trap Explosions
Here's something I didn't hear about until my fourth year of college. I heard whispers of this phenomenon in my third year, but knew nothing of it until fourth year. It's called a liquid oxygen explosion. When I first heard the term I was thinking it was an expansion of a liquid to a vapor that would burst the container much like my liquid nitrogen bomb described in the previous section - and I was partially right. I was wrong to assume that ignition does not occur.
Liquid oxygen is somewhat warmer that liquid nitrogen, but only a little. This means that if you put a tube in liquid nitrogen and blow air through it, you would literally condense oxygen out of the air in the tube. You would have a tiny amount of liquid oxygen. Since it's so cold, I figured nothing would ignite it. WRONG!
Oxygen is the second-strongest oxidizing element known to man. Elemental fluorine is the only element stronger, which you never find elemental fluorine anywhere except in laboratories (it didn't even exist until the 1900s). Anyway, vaporized oxygen takes up five gallons for every 32 grams. 32 grams of liquid oxygen takes up a couple tablespoons. Quite the density difference, eh? Liquid oxygen means you're putting the oxidizing power of a lot of air into a tiny space. That's one of the reasons it's the prefered fuel of choice for the shuttle launch. This means that anything that burns will burn much quicker and more violently in the presence of liquid oxygen than in air.
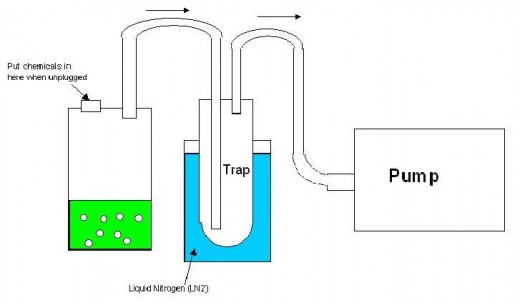
I had a close call with one of these explosions. There's a thing called a solvent trap in advanced chemistry. You hook a hose up to a vacuum pump to alter atmospheric conditions in reactions. However, the solvent will eat the fittings in the pump and destroy the pump, which is expensive. So you put part of the hose in liquid nitrogen and freeze out the solvent before it gets to the pump. This is a solvent trap. See the sketch below. Most solvents are flammable. In fact the majority of carbon-based solvents are incredibly flammable. Acetone (my favorite), hexane, octane, alcohol, ether, esters, chloroform, benzene... just to name a couple. Anyway, a little bit of air leaks into your system and passes through this solvent trap. The oxygen will condense in the trap if the conditions are right. This means that you have strong oxygen soaking into solvents that are incredibly flammable. One spark would detonate it with a force that would turn the glass into sand traveling thousands of miles per hour.
What happened to us is that we turned off the pump and left all the valves closed. We removed the trap from the nitrogen and didn't leave a way for the vaporizing oxygen to vent pressure from inside the trap. Bad idea, and it was a genuine accident. The pressure grew inside until it blew the trap off and shattered the glass all over the counter. Suddenly dozens of people rushed into the room to see if a disaster had occurred. If there had been an open heat source, I would have ended up in the hospital (and there were heat sources used all over that place that day. I just got lucky they were all turned off in that area.).
So imagine a place that could potentially detonate gallons of this stuff at a time. It would look like Osama got a hold of the place. Chemical factories that aren't careful enough have experienced shit like that before.

Fireballs in Hollywood
We all love to see the fireballs in explosions in Hollywood. Thing about explosions is... it takes a special case to see a fireball explosion like that. It doesn't happen like that in most explosions.
Take C4 or nitroglycerin or TNT. The oxidizing part of the molecule is attached to the reducing part of the molecule, which makes it a very unstable explosive. In C4 the balance of oxidizer to reducer isn't right, so they add a little powdered aluminum to balance it and to make it burn hotter.
Now a the light in a fire comes from electrons moving from reducer to oxidizer. When you burn wood, the fire extends a few inches into the air because unburned hydrocarbons (reducers) float higher before they encounter an oxygen molecule to transfer their electrons to.
Have you seen a cutting torch adjusted properly? There is a point where the fire is just at the tip - this is because the oxygen and the fuel are perfectly mixed and burn only at that one point, leaving no unburned hydrocarbons to carry the flame farther out - at least not a significant flame farther out. The reducer-oxidizer ratio is perfect (redox).
So when you see a conventional explosive detonate, it's usually a flash from the initial redox reaction taking place, but the expansion beyond that involves no yellow fireball - just a dust ball. That's why in war movies the explosions usually don't involve yellow roaring balls of fire.
I'm not saying the yellow roaring balls of fire don't exist. I'll tell you where they do exist: when you have an imbalanced ratio of reducer to oxidizer. If the explosive has too much reducer then the explosion will push the left-over reducer out into the atmosphere, where it will ignite with the atmospheric oxygen and the chemical reaction will take place much like a fireball you always imagine. Gasoline is a great reducer and the liquid contains no oxidizer. Only the vapors mix with the air and burn. In a car bomb, the bomb itself has a balanced reducer-oxidizer ratio, but blows that gasoline in the tank out into the atmosphere and ignites it at the same time, giving you a great Hollywood fireball. I love gasoline for its fireball effect.
Why the fireballs in nuclear explosions? Well, nuclear explosions contain tens of millions of degrees in temperature. When in a nuclear explosion, the initial blast on the ground is from the top layers of everything vaporizing quickly and then the rush of the explosion blows what's left away. Then you have Indiana Jones staring at the rising mushroom cloud that's still very much orange and yellow. The reason the fireball has a lingering fire look to it is because it is so hot. The electrons in the cloud are still moving from atom to atom and giving off light as they do so. It's not until the fireball has cooled to a point where all the electrons settle into their places that the fireball looks more like a dust/ash ball.

Rocket Fires
I was explaining above that if the reducer-oxidizer ratio was balanced in a reaction, the fire would take a very small space and be over very quickly. This is partially true. Sometimes if the fire is too hot, the electrons won't settle until it cools a little - and the electrons settling is what causes the light to be given off in a fire. So in a shuttle launch, the fire stretches out behind the rocket for a while because the fire is so hot that the electrons won't settle until it's cooled a bit. That and it also takes a little time for the hydrogen and oxygen fuels to mix to the proper ratio, but that's a small fraction of the reason a fire stretches behind a rocket. That's another reason perchlorate explosions create a bit of a fireball - it's so hot the electrons take a little extra time to settle and in that extra time, the explosive has expanded to the size of the fireball you see.
Freezing Hot Water
Have you ever heard that hot water will freeze before cold water? This is not accurately put! It's possible to freeze hot water, but the conditions have to be perfect, otherwise you are talking jibberish and look like a fool.
Imagine the following situation: you place a little hot water in a freezer next to a lot of cold water. When you open the freezer ten minutes later, the hot water will have frozen and the cold water will still be liquid. How is this possible? Because it takes energy to melt ice, or the lack of energy to freeze ice. Hot water has more energy per amount than cold water, but if there's significantly less hot water there's less energy total. That is why one cup of hot water will freeze before a gallon of cool water.
But what if it's the same amount of water? It can still be done, but carefully. When you place hot water next to water that is slightly cooler, and they both have the same amounts, the hot water will freeze first. This is because the hot water is evaporating over time quicker than the cooler water next to it. Even though you started out with the same amount of water in both cups, you will end up with less hot water that you have cool water - and the one with less water will freeze first.
Cool trick, eh? I love science. If science was a woman, I'd be in bed with her all the time. MMM!







