The Chemistry ABC
The Chemistry Alphabet
Chemistry, like all of the other sciences, is filled with it's own language. Key terms are dotted throughout textbooks, but also in press releases and news bulletins. This can make it very difficult to understand just what a chemist is actually talking about. Here we look through some of the most important terms found in GCSE (14-16yrs) chemistry, their meanings and some interesting facts.

A is for Acid
Acids are substances that donate hydrogen ions in reactions. It is this that causes them to burn your skin. Alkalis (soluble bases) donate hydroxide ions instead
B is for Bond
A bond is how two or more atoms connect to each other. There are several types:
- Ionic
- Covalent
- Metallic
The type of bonding usually dictates the behaviour of the resulting molecules
C is for Compound
A compound is two or more different elements chemically bonded together. They cannot be separated by physical means, instead requiring a chemical reaction to split them apart. Remember, the atoms must be different to make a compound. Oxygen has two atoms but is still considered an element as the atoms are identical.
D is for Diffusion
Diffusion is the movement of particles from a region of their high concentration to a region of their low concentration. This is entirely due to chance and the random movement of the particles. Eventually diffusion will result in the particles being evenly distributed within a container. Only liquids and gases can diffuse, as their particles are free to move and not held in a rigid structure (like solids).

E is for Element
Put simply (and wrongly) an element is the simplest substance there is. There are 118 different elements (at last count) and are all recorded in the Periodic Table. Each Element has a unique number of protons in the nucleus of the atom. It is this that gives the element its properties. A gold bar is an element because it is made up of only gold atoms. These atoms cannot be broken apart into smaller atoms. This is what makes it an element
(OK yes I know you can break an atom into subatomic particles, and these into even smaller elementary particles, but for the purposes of this explanation, this is good enough!)
F is for Fluorine
Fluorine is the most electronegative element on the periodic table - this means it is the best element at grabbing electrons from other atoms. This makes fluorine very very reactive. Hydrofluoric acid (an acid made with fluorine) is so corrosive it can dissolve glass...making it difficult to store! On the other hand, fluoride ions are actively added to the water supply of many countries as this ion strengthens our teeth!
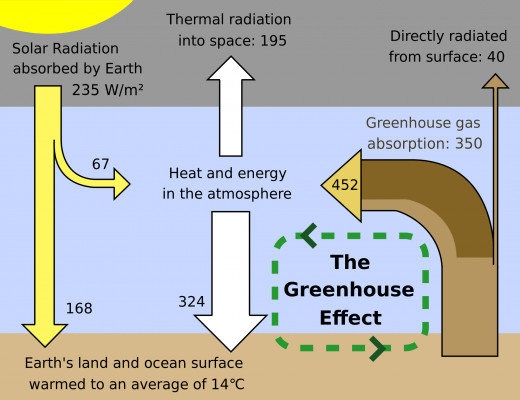
G is for Greenhouse Gas
Greenhouse gases are gases that trap heat in our atmosphere. The most well known of these is carbon dioxide (which contributes up to 26% towards the greenhouse effect. There are many, much more potent, greenhouse gases that are finding their way into the atmosphere. These include:
- Water vapour - naturally occuring and contributes anywhere between 36 and 72% to the greenhouse effect
- Methane - contributes up to 9%. This gas is at much much lower levels in the atmosphere than carbon dioxide, but is many times more efficient as a greenhouse gas
- Nitrous Oxide
- Ozone - contributes up to 7%
It is important to realise that, without any greenhouse gases, our planet would be far too cold for life to exist. Current temperatures are not even the highest they have ever been on Earth. The problem is the rate of temperature increase. It is happening too quickly for life on Earth to adapt. If you want a look at what runaway greenhouse effect can cause, look no further than Venus - the hottest planet in the Solar System
H is for Halogen
Halogens are found on the right of the Periodic Table, in group VII. This is the only group in the Table that contains elements found in all three traditional states of matter at room temperature and pressure:
- Chlorine (gas)
- Bromine (liquid)
- Iodine (solid)
- Astatine (solid)
All of the halogens have 7 electrons in their outer shells, giving them similar properties.
I is for Ions
Atoms have an equal number of protons and electrons. Ions are formed when an atom gains or loses an electron.
- Electrons are negatively charged;
- protons (sub atomic particles in the nucleus of an atom which determine the properties of the atom) are positively charged.
- When an atom loses an electron, it is one negative charge 'short' and gains a positive charge (more positives from protons than negatives from electrons.)
- When an atom gains an electron, it has one more negative charge and so becomes a negative ion.
J is for...well...nothing
O.k this is a cheat, but J is the only letter not used in the Periodic Table

K is for Potassium
eh? What?
The Periodic Table uses symbols that are international. This means that equations written by a scientist in Japan can be understood by scientists in France or Spain or Sweden or USA. These symbols, therefore, do not always make sense in English. Potassium is derived from the old-English word Potash. Potash in Latin (the dominant scientific language at time of discovery) is 'Kalium,' meaning alkali. It was called alkali because, when placed into water, potassium makes an alkali. Indeed, potassium is in group one of the periodic table - one of the 'alkali metals,'
L is for Lithosphere
The outermost layer of any rocky planet - the lithosphere is where we live, work and play.
M is for Metal
Metals make up most of the Periodic Table. They are (usually)
- Shiny
- Ductile
- Malleable
- Conductors of both heat and electricity.
Not all metals are hard - some of the Alkali Metals (metals in group I of the Periodic Table) can be cut with a table knife.
N is for Neutral
At room temperature and pressure, neutral pH is 7. This means the solution is neither acid nor alkali. It donates neither protons nor hydroxide ions. It accepts neither of these.
O is for Oxidation
At it's most basic, oxidation is the act of adding an oxygen atom to another atom during a chemical reaction. It can also be defined as:
- The loss of electrons
- The increase of oxidation state.
Oxidation reactions are always accompanied by their opposite, the reduction reaction (gain of electrons or decrease in oxidation state). This is why such reactions are referred to as redox reactions. To remember which is which. just think of OILRIG - Oxidation Is Loss, Reduction Is Gain
Elements Song - Tom Lehrer
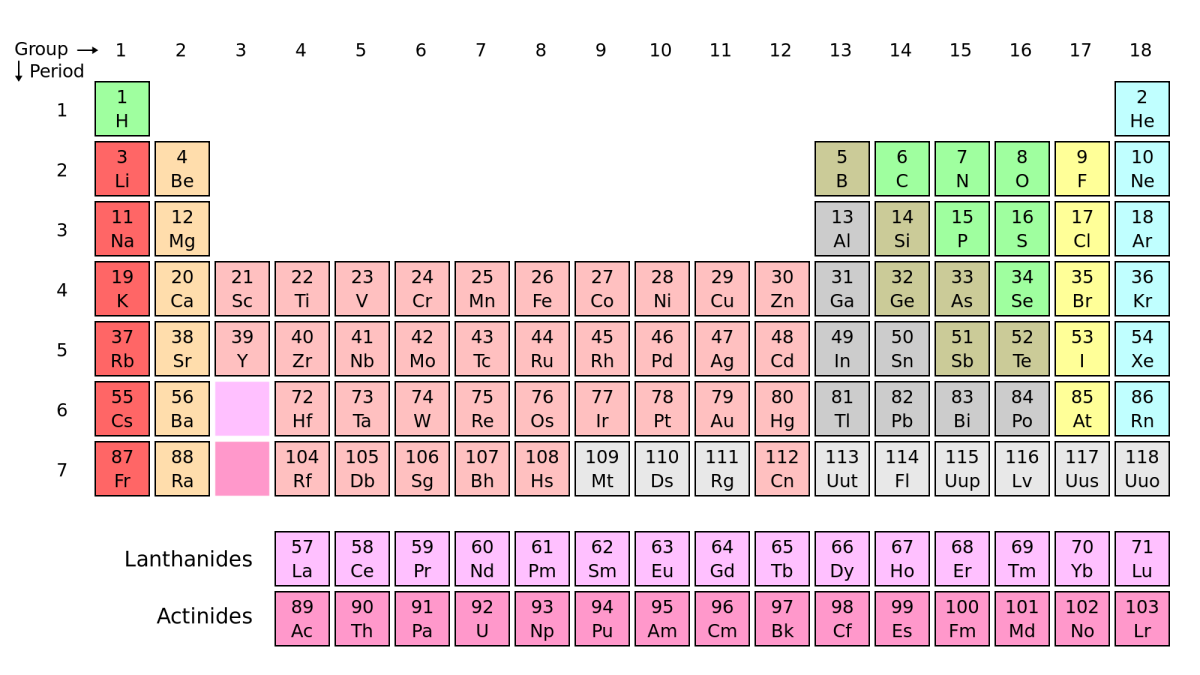
P is for Periodic Table
The Periodic Table is the current method of organising all 118 elements according to the number of protons in the nucleus of each atom. The Table was devised by Dmitri Mendeleev in 1869. This was a revolutionary breakthrough in organising elements. Until this point, chemists could arrange known elements by different characteristics, but their patterns were disrupted when a new element was discovered. Mendeleev's table not only allowed for un-discovered elements, but predicted their mass and properties!
The table contains only elements (no compounds or mixtures) and is arranged in two main ways:
- Groups - these go up and down they show strong patterns in atomic radius, ionisation energy and electronegativity. All elements in a group have the same number of electrons in their outer shell.
- Periods - these go across the table. Atomic radius decreases across a period. As you move across a period, the number of protons in the nucleus of the atom increases by one each time.
Q is for Heat Energy!
q=mCΔT to be precise! This equation shows the amount of heat energy in a system:
- q - energy
- m - mass (J)
- C - specific heat capacity
- ΔT - change in temperature

R is for Reaction
Chemistry is all about reactions. Without reactions, stars would not shine, fires wouldn't burn and no life could exist. Chemical reactions occur when:
- Two or more atoms create a bond between them
- A molecule breaks one or more of it's bonds.
Reactions are described using equations. These can be word equations or symbol equations. These tell you what is happening to the atoms in a reaction. For example, combustion of methane can be described as:
WORD: Methane + Oxygen → Carbon Dioxide + Water
SYM: CH4 + 2O2 → CO2 + 2H2O
Each symbol is taken from the Periodic Table. The large numbers show how many of the subsequent molecule/atom there are in the reaction. The subscript numbers show how many of the preceding atom are in the molecule. The large numbers are important to make sure that there are the same number of atoms on both sides of the reaction: What goes in, MUST come out.
S is for Solutions, Solvents, Solutes and Solubility
A personal Bug-bear of mine. So many of my students do not know the difference between these basic terms. SO...here we go:
- Solution - Mixture of a substance (solute) dissolved in a liquid (solvent);
- Solvent - Liquid that does the dissolving to form a solution;
- Solute - a substance that dissolves in a liquid to form a solution;
- Solubility - describes how easily a substance will dissolve in a liquid (usually water).
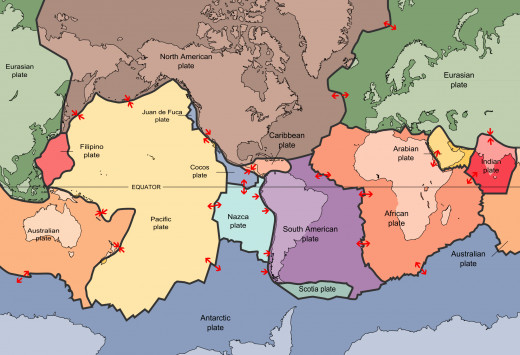
T is for Tectonic Plate
O..k.. Strictly this should be geology, but it is a vital part of the recylcing of the Earth's chemistry. A tectonic plate is one of the large pieces of the Earth's crust that gradually moves across the surface of the mantle. They float on top of the much denser mantle and move very slowly on top of a conveyer belt of convection currents at a rate of about 2.5cm per year.
Tectonic plates are a relative newcomer to our theory of how the Earth is structured. Alfred Wegener published his theory of continental drift in 1914 after he noticed that the continents seemed to fit together like a jigsaw. As Wegener couldn't provide any explanation for how these plates moved, the theory was disbelieved by the wider scientific community until the 1960s
U is for Universal Indicator
Universal Indicator is a substance that changes colour depending on the pH of a substance. The most accurate Universal Indicators correspond to the different colours of the pH scale.
V is for van der Waal Forces
These intermolecular forces are much weaker than covalent bonds but are key in determining many traits of substances, including their solubility. Van der Waal forces describe all of the attraction (or repulsion) between substances that are not described by covalent bonds, hydrogen bonds, or electrostatic interactions.

W is for Water
During the Middle Ages, alchemists spent their entire lives looking for a universal solvent (when they weren't trying to create a Philosopher's Stone) - a liquid that would dissolve any chemical. They made many weird, wonderful and useful creations, but none that could dissolve any substance on Earth.
The closest thing we have is water - this weird molecule dissolves more solutes than nearly any other fluid. Water has many weird properties:
- It's solid state is less dense than it's liquid state
- The densest point of water is 4°C - it gets less dense both above and below this temperature
- It is vital for all known forms of life
- Has unusually high surface tension due to a weird number of hydrogen bonds it can form
Chemistry Quiz
view quiz statisticsX is for Xenon
Xenon is the only element in the Periodic Table to have X in it's symbol. Xenon is used in lasers, anaesthetics and flash lamps
Y is for Yield
The amount of product made in a reaction
Z is for Zinc
O.k maybe I am cheating with yet another element from the Periodic Table, but I am stumped! Zinc is a metal. Zinc is actually extremely important in biology - zinc deficiency affects millions (if not billions) worldwide. This can cause a huge number of diseases and is thought to contribute to the deaths of 800,000 children every year. The reason zinc is so important is that it forms a key part of many of our enzymes. Without fully functioning enzymes, many of our vital chemical reactions do not work or work too slowly.