Future Scientific Inventions I am Interested in: Gene Therapy for Cancer, Hepatitis B Test
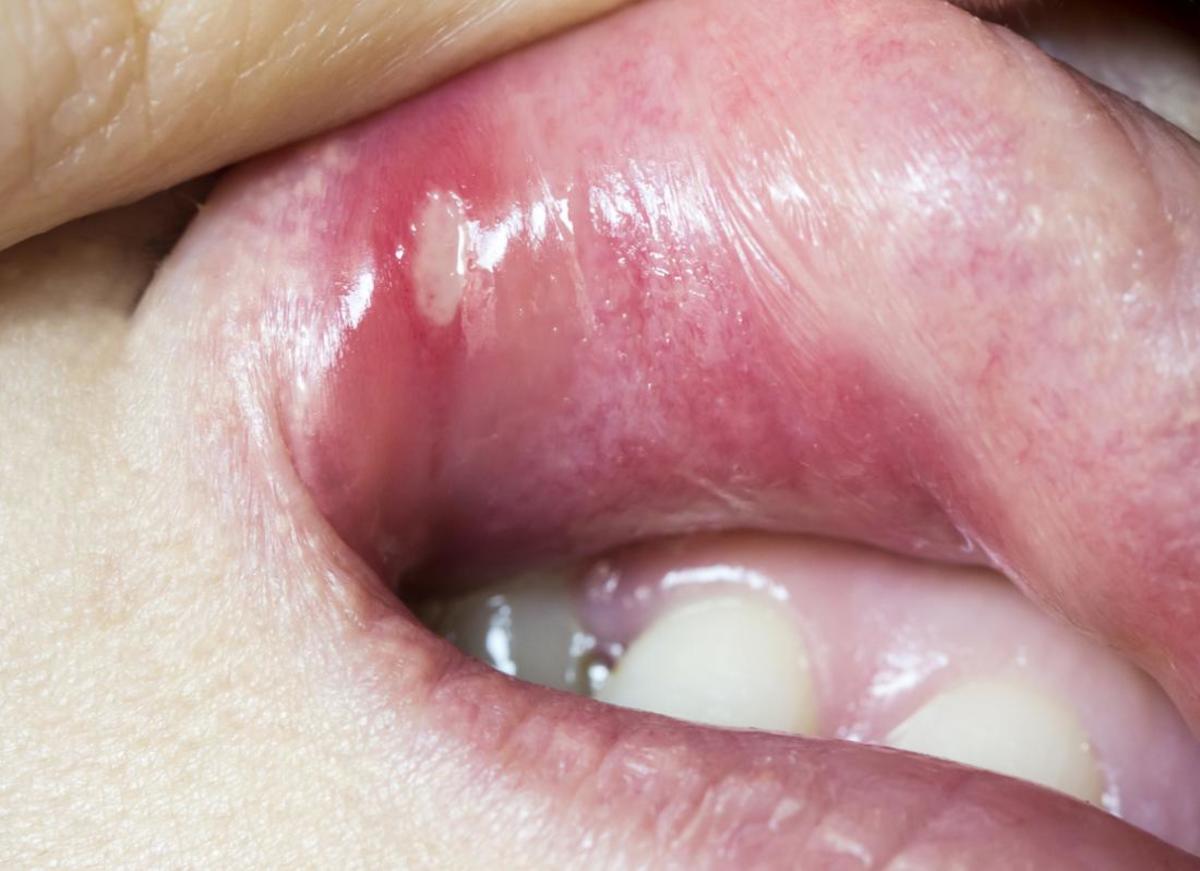
Infusion chelation therapy for heart disease is similar to that for hepa B. This picture was taken in the clinic of Dr. Estuita, MD.

Scientific inventions I would like to see and why
Gene therapy for cancer
This therapy had been tried on mice and found out that it works. However, it has yet to be proven that it works on human beings. It only has to go through the standard three phases of trial for drugs or protocols.
Gene therapy is highly specific, unlike the currently used chemotherapy. Inducible nitric oxide, a free radical, is delivered precisely on cancer cells. Inducible nitric oxide kills cancer cells. It also kills healthy cells. However, since its delivery to cancer cells is very specific, it does not land on healthy cells. Therefore, no healthy cell is killed with the use of gene therapy for cancer. So it has no side effects. I have two Hubs on this topic, "A new treatment for cancer: gene therapy," and "Chemotherapy and gene therapy compared."
Gene therapy for cancer may be considered as alternative medicine because it openly admits the use of free radicals. Conventional medicine ignores free radicals as cause of disease or as agents of treatment. It mentions chemotherapy but does not mention the active ingredient that are free radicals.
Cancer is a top world killer, next to heart disease.
More sensitive test for hepatitis B virus
Hepatitis B virus (HBV) that bring on hepatitis B cause liver inflammation, cirrhosis and liver cancer. They are more dangerous than the human immunodeficiency virus (HIV).
Some countries, like United Kingdom, have standards on HBV, meaning the minimum virus load of a person that is considered as non-infectious. The standard is 1000 to 1500 copies per milliliter of blood.
HBV consists of surface protein not attached to DNA (deoxyribonucleic acid), and infectious particle. HBV is a DNA virus, other virus are RNA or ribonucleic acid. DNA contains the information code in the production of protein, thus the whole human body; RNA is a messenger that delivers the information code (Cummings, M. Human Heredity. 2009).
A surface protein is empty, meaning it does not contain DNA. HBV produces more surface protein than those with infectious particles, that is, surface protein with DNA. There are 50 trillion surface protein in a teaspoon of blood. There are 500 million infectious particles in one teaspoon of blood (Offit, P., MD and L. Bell, MD. Vaccines: What Every Parent Should Know. 1996: 77-79).
If you compute for the ratio of empty surface protein to infectious particle, you get 100 thousand surface protein is to 1 infectious particle.
By interpolation you can see that even with a virus HBV load of 90 thousand a person is probably non-communicable. However, that is by means of mathematical interpolation. What is to be ascertained is that this virus load is not communicable by the use of a test.
Probably, the Koch postulate can be used to test if HBV load of 90 thousand is infectious or not.
There is now a gadget being used that can give the result of blood test for HBV or hepatitis virus for that matter. In 4 to five days the doctor can get the read out, according to Dr. Arturo V. Estuita, a Filipino internist and chelationist who is treating hepatitis B with infusion chelation therapy.
However, it appears that that test does not distinguish whether a test is false positive or not. That is, if the gadget gives a positive result does it mean that the virus load is infectious?
It is highly probably that the test reacts to the hepatitis B antigen. However, an antigen is produced by the immune system for an empty surface protein and an infectious DNA particle.
That is proven by the hepatitis B vaccine itself.
New entries as of August 15,2013
To recall, the hepatitis B vaccine consists of surface protein only. When it is injected to a healthy person, his immune system will react to it. That is, the immune system does not care whether it is empty or infective: containing DNA. The immune system will produce B cells, among others. The person will produce antigens and antibodies against the empty surface protein - with fever and fatigue among other symptoms. When the empty surface proteins will have been conquered suppressor cells will stop the production of B cells like macrophage. (In a way the immune system is being fooled.) Most of the B cells will die off but some will remain alive as Memory cells. When new empty surface proteins or infective hepatitis B virus get into the same person, the Memory cells will induce the immune system to eliminate these foreign bodies. That is how vaccination works.
The killed poliovirus vaccine invented by Dr. Jonas E. Salk and his team works similarly. The poliovirus had been killed by formaldehyde. This chemical also had hardened the viral coat and maintained its size and shape. The killed poliovirus in Salk vaccine are incapable of replication or multiplication or of infection or both. When Salk vaccine is injected, the immune system multiplies the population of macrophage, produces antigens and antibodies against the killed virus. When the foreign invaders will have been "conquered" B cells will die off but some of them will remain as Memory cells. The fellow is now immunized.
The oral polio vaccine (OPV) invented by Dr. Albert Sabin is different. It consists of live but weakened poliovirus to which the immune system responds. However, some of these weakened virus may mutate and become infective. That is why a fellow who had taken OPV can infect another person who may get sick of polio (Kluger, J. Splendid Solution. 2009).
How do we know that a virus load of, say, 12,000 is infectious or not? One way is to see if the virus load increased in population after the treatment with infusion chelation, for example. If the population increased, it means that the viral load has infectious particles. If not, it means that the viral load consists of empty surface proteins only, One book says that the test for increase in population of viral load should be done in 4 to 6 months after the treatment by infusion chelation therapy, for example.
Dr. Estuita outlines his ways of determining whether a fellow has empty surface protein or infectious particles as follows:
"On (a patient whose name cannot be mentioned because of confidentiality) return I want to check for HBV proliferation or replication and infectivity (HBeAG), immune response (HBsA) and HBV load."
"Return" means that Dr. Estuita had earlier treated the patient of hepa B. "HBV" means hepatitis B virus. "Proliferation" or "replication" means multiplication, that is the population of HBV increased. "Infectivity" means the HBV caused hepatitis B. "Immune response" means that the person produced antigens, antibodies, and B cells and Memory cells. "HBV load" means the virus load increased, say, from 10,000 copies per milliliter to 50,000 copies per milliliter.Or the population remained the same or it decreased.
The immune response may show false positive. That is, the immune system shows a positive response but the virus load remained the same or it decreased. That means the patient had been cured by the earlier treatment Infectivity, like recurrence of hepatitis B or inflammation of the liver and other diseases like malignancy in the liver or cirrhosis, would be negative. End of new entries.
Outlook for people with hepa B virus
A lot of people has been infected with hepatitis B virus as shown by current laboratory test. As a result, they cannot get jobs. A woman shown by test as positive to hepatitis B cannot have a baby because this hepa B virus is transmitted by body fluid. Unless the man is willing to get infected by hepa B virus. The newborn baby can by immunized by vaccination.
Review of standards a must
For another thing, the world standard on hepatitis B load that is considered non-infectious should be reviewed and modified. It is highly probable that the present standards are not attainable, and attainment may not be necessary.
Such a review and modification of international standards will benefit other members of the family of the fellow who is thought of having an infectious HBV load. They are always wary of being infected when in fact the suspected carrier is not infectious at all. The new standard will also impact on treatment and cost of treatment.
A review will benefit the medical profession as well. Doctors would not be chasing standards that are unattainable or unnecessary or both.
New entries as of April 22,2014
Indicators of the need to go for hepatitis B treatment
I have found in the Internet what I have been looking for. A worldwide standard as to the threshold of viral load.
Taiwan conducted a study for 15 years to find bases for the treatment of hepatitis B (Steven-Hub Han. Hepatitis B - Treatment and Consequences. Youtube, April 20,2014). It was found that the accurate indicators are viral load and ALT (alanine amino transferase). The threshold levels are: viral load less than 20,000 international unit/ml and ALT at less than 1 x ILN. At these levels, just monitor the disease. No treatment. That means the virus does not replicate and the person is not communicable.
At a level higher than this threshold, give treatment. I discuss this topic in my Hub "Accurate indicators of the need to go for hepatitis B treatment."
.