Magical Gold Nanorods
Abstract
Facets, gold nano hollow cages, gold nanohorns, gold nanocubes, gold nanoparticles and nanorods. One of the most promising is the gold nanorods. Gold nanorods are adjustable due to their unique optical activity (longitudinal and lateral plasmon resonance absorption peaks, adjustable range from visible light to near-infrared region), long aspect ratio, easy surface modification and good biocompatibility. It has broad application prospects in the fields of nanobiology and biomedicine. The synthesis and surface modification of gold nanorods directly determine their physicochemical properties, which in turn affect their biocompatibility and their application in biomedicine. This paper reviews the controllable preparation methods, surface controllable modification methods of gold nanorods and their application in nanobiology and biomedicine. The controllable synthesis methods of gold nanorods include template method, electrochemical method, photochemical method and seed crystal method, and the controllable surface modification methods include ligand replacement method, inorganic material coating method and multi-stage modification method. Gold nanorods have made new advances in nanobiology (including molecular probes, biosensing, and bioimaging) and biomedical applications (including drug carriers, gene carriers, and photothermal therapy). Finally, some bottlenecks in the process of biological application of gold nanorods, such as the need for specific recognition and the quantum yield of fluorescence, need to be improved. It is also proposed to introduce chiral molecules or smart polymers into the surface of gold nanorods for controlled modification, in order to enhance their specific recognition ability and increase the fluorescence quantum yield, which provides a new idea for the development of gold nanorods.
Synthesis of Gold Nanorods
Electrochemical method
The first method to synthesize high-quality gold nanorods was the electrochemical method, which later became a pioneer in seed growth. In the 1990s, gold nanorods were completed under a two-electrode cell, as shown. The gold plate serves as the anode and the platinum plate serves as the cathode, both of which are embedded in the electrolytic cell. Nanorods with a certain aspect ratio can also be obtained by using a mixed surfactant solution of cetyltrimethylammonium bromide (CTAB) and tetrabromododecyl ammonium (TDTAB) as the electrolytic cell solution. During the electrolysis, gold ions move from the anode to the cathode in the form of , and finally to the gold element at the cathode. The silver plate is gradually embedded in the electrolytic cell to control the aspect ratio of the gold nanorods, wherein the consumption of silver ions is achieved by a redox reaction between the gold ions and the silver plate.
A small amount of acetone and hexane are also added to the reaction system to promote the formation of a rod-like structure, and the whole process is in an ultrasonic state. Among them, CTAB can not only prevent the aggregation of gold nanoparticles, but also act as an electrolyte. TCAB can also induce the formation of AuRNs. The aspect ratio of AuRNs can be controlled by the ratio of TCAB and CTAB. The advantage of the electrochemical method is that the morphology of AuRNs is easy to control and uniform in size.
Seed growth method
The method is the most common method for synthesizing gold nanorods. The method is simple in process and can synthesize high-quality, high-yield gold nanorods with easy shape and shape control. MURPHY replaced the gold plate in the electrochemical process with tetrachloroauric acid as the gold source, and replaced the redox reaction in the electrochemical with the weak reducing agent ascorbic acid (AA) and silver nitrate, and the original mixed surfactant system. CTAB and TDTAB were replaced by one of CTAB, but the original acetone and hexane were retained. In this synthesis method, low concentrations of AA did not reduce tetrachloroauric acid to a metallic state, but existed as Au-. However, after adding 3 nm of gold particles (as a seed crystal), the growth solution is catalyzed by the surface of the gold particles, and Au- is oxidized to a simple substance of gold, gradually becoming a spheroidal shape, and finally a rod-like structure is formed. It has been confirmed that the less seed crystals are added, the higher the aspect ratio of gold nanorods, but the yield of gold nanorods is still low, and more spherical particles are produced. The aspect ratio of the gold nanorods can be controlled by adding a seed solution to a different growth solution.
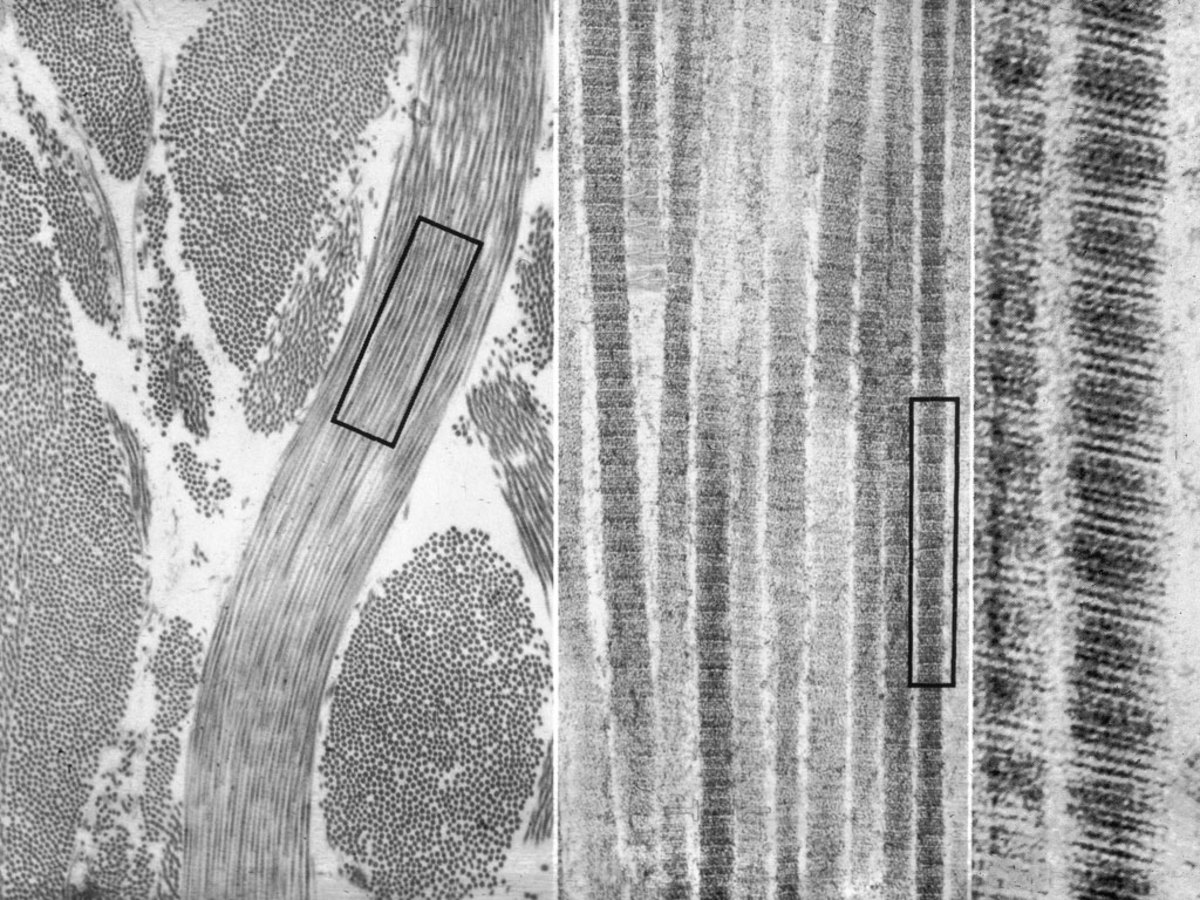
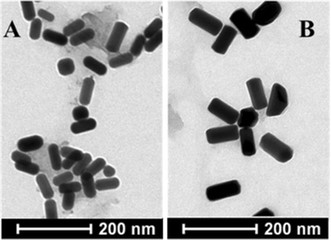
In 2003, EL. SAYED et al. improved this method, greatly reducing the yield of spherical particles and increasing the yield of the rod-like structure as shown. Two systems were created by this method. One is to use a high concentration of CTAB to coat the seed crystals, and a certain concentration of silver nitrate will yield a rod yield of more than 95%, and the aspect ratio can reach 4.5, the longitudinal surface. The plasmon resonance absorption peak wavelength can reach 850nm, as shown in the figure; the other is the mixing of CTAB and benzyl dimethyl ammonium chloride (BDAC) as a surfactant, the gold nanorod obtained by this system. The aspect ratio can reach 10, and the longitudinal surface plasmon resonance absorption peak wavelength can reach 1300 nm, as the picture shows.
The position of the longitudinal surface plasmon resonance absorption peak can be adjusted to some extent by controlling the concentrations of silver nitrate and tetrachloroauric acid. The position of the absorption peak shifts toward the long-wave direction as the concentration of silver nitrate and tetrachloroauric acid increases, but the concentration increases to a certain extent, but the movement is inhibited. JIANG et al. found that the position of the longitudinal surface plasmon resonance absorption peak of each batch of synthesized gold nanorods is different, which is related to many factors, especially the relationship with the seed crystal. Since the synthesis of the seed crystal requires the addition of sodium borohydride, and the amount of sodium borohydride added is slightly increased, the impurities are greatly increased. This rule can be easily distinguished in the color of the seed crystal. In addition, changes in the ratio of tetrachloroauric acid to AA lead to changes in the position of the longitudinal surface plasmon resonance absorption peak, which has been found to result in shorter gold nanorods and lower absorption peaks. Recently, MURRAY et al. reported a new technology for the synthesis of gold nanorods - the use of aromatic additives and low concentrations of CTAB. This method has been proven to give high aspect ratio gold nanorods with fewer impurities.
Although the seed growth method can produce higher yields of gold nanorods, many parameters such as seed solution concentration, aging time, temperature, pH and CTAB concentration can significantly affect the shape and length to diameter ratio of gold nanorods, yield and location of the absorption peak. To repeat the synthesis of a gold nanorod of a certain aspect ratio, the consistency of all key parameters must be maintained.
Template method
The templating method mainly utilizes the pores of the porous alumina film, reduces the gold atoms in the pores, and allows the deposition to be limited until the pores are filled, and then dissolves the aluminum oxide film with sodium hydroxide and then obtains the dispersion with ultrasound. AuNRs. In 1994, Martin et al. used the template method to synthesize AuNRs for the first time. They reduced and deposited Au into the pores of the porous alumina film by electrochemical deposition, and then dissolved the porous alumina film (template) and removed the impurities generated during the reaction to obtain AuNRs. In the process of synthesizing AuNRs by template method, the growth of AuNRs is bound by the template pore size. Therefore, the size of the template pores directly determines the diameter and length of AuNRs. Therefore, different sizes of AuNRs can be synthesized by different templates. The advantage of the templating method is that the aspect ratio of AuNRs can be controlled by pore size, pore length, and electrochemical deposition time.
Optical Properties of Gold Nanorods
Metal nanomaterial has superior optical properties relative to bulk metallic materials. When light is incident on the surface of the metal nanoparticle, if the incident photon frequency matches the vibration frequency of the metal conduction electron, the nanoparticle will strongly absorb the incident light, thereby causing localized surface plasmon resonance (LSPR) phenomenon. . The interaction between the optical field and the free electrons in the nanoparticles causes the free electrons to separate from the metal nucleus, while the Coulomb repulsion between the free electrons acts as a "recovery force", which in turn causes the free electrons to move in the opposite direction, thus causing free electrons. The collective oscillation produces an LSPR. Metal nanoparticles of different sizes, shapes or materials exhibit different absorption capacities and also exhibit different colors. For gold nanorods, the anisotropy of their structure produces two absorption bands in the absorption spectrum. Lateral surface plasmon resonance of gold nanorods has been found to be insensitive to changes in the size of the gold nanorods and changes in the surrounding refractive index, whereas the longitudinal direction is very sensitive. Therefore, the nature of longitudinal surface plasmon resonance is highly dependent on the size and shape of the gold nanorods and the dielectric constant of the surrounding medium. With the change of the aspect ratio, the position of the longitudinal absorption peak can be shifted in the wide band of visible light near infrared, and the position of the lateral absorption peak is smaller. Therefore, gold nanorod solutions of different aspect ratios can exhibit different colors such as blue, brown and red.
For metal nanorods, GANS predicts that for small, ellipsoidal, dipole-like nanoparticles, surface plasma forms can be divided into two completely different forms due to differences in surface curvature and geometry. These small sticks are generally considered to be ellipsoids when interpreting the optical properties of gold nanorods. Therefore, GANS theory can be applied to describe the optical properties of gold nanorods. It can be seen from the spectrum of the test results that weak absorption of lateral surface plasmon resonance and strong longitudinal surface plasmon resonance appear in the visible and near-infrared regions, respectively, where the longitudinal absorption peak position follows the long diameter. The increase in the ratio is red-shifted and the lateral movement is small. Both absorption peaks shift with increasing dielectric constant of the medium, but the red shift of the longitudinal absorption peak wavelength is much larger, indicating that longitudinal surface plasmon resonance is more sensitive to changes in the dielectric constant of the surrounding medium.
The graph shows the relationship between the absorption spectrum of the gold nanorods and the dielectric constant in the case where the aspect ratio is constant.
Surface modification technology of gold nanorods
When applied to biochemical and biomedical fields, gold nanorods are required to have good biocompatibility and colloidal stability. For the gold nanorods synthesized by the seed growth method and the electrochemical method, the surface is covered with a positively charged CTAB bilayer. In the preparation of gold nanorods, CTAB not only acts as a structure-directing agent when controlling particle formation, but also prevents aggregation of gold nanorods, acting as a stabilizer and a protective agent. However, CTAB-coated gold nanorods exhibit good stability only in lower pH solutions. When transferred to other solutions, such as organic solvents, gold nanorods are prone to aggregation, and CTAB-coated gold nanoparticles The stick also inhibits the endocytosis of the cells. In order to improve the stability and biocompatibility of gold nanorods and avoid the aggregation of gold nanorods, suitable surface modification is a key problem that gold nanorods need to solve in biological functionalization and biological system applications.
At present, there are various methods for surface modification of CTAB-coated gold nanorods. Generally, there are two ways to modify the surface of gold nanorods: one is ligand exchange, which refers to surface modification molecules, usually small molecule compounds, which are bonded to the surface of gold nanorods by chemical bonds; the other is surface coating. , refers to the direct coating of gold nanorods with organic or inorganic materials (usually polymer materials, biomolecules, surfactants, etc.).
Ligand exchange method
In this method, a CTAB bilayer is replaced by a mercapto compound as a ligand, and a mercapto compound is firmly bonded to the surface of the gold nanorod by Au-S covalent bond. The surface-modified gold nanorods can be widely used in biochemistry and biomedical fields nanotube after being functionalized by chemical or biological means. In addition, the removal of CTAB molecules can effectively reduce the biological toxicity of gold nanorods. At the same time, the probability of gold nanorods gathering is greatly increased due to the loss of the protection of CTAB molecules by the gold nanorods during the modification process. Therefore, the modification process requires strict control of experimental conditions to avoid aggregation of gold nanorods. The mercapto compound polyethylene glycol (mPEG-SH) is a commonly used ligand in the surface modification of gold nanorods. Since polyethylene glycol (PEG) is a water-soluble polymer, gold nanorods can be modified in water. In addition, PEG has high stability in some organic solvents and is also widely used in vivo. HAFNER et al. replaced the CTAB molecules on the surface of a part of the gold nanorods with mPEG-SH to obtain stable gold nanorods. However, the coupling step of PEG molecules is cumbersome and time consuming, so it is very important to find a biological small molecule and the biological modification is simple and easy to operate.
Many studies have indicated that mercapto undecanoic acid (MUA) can completely replace CTAB molecules and achieve surface modification of gold nanorods. MUA is a mercapto compound with a carboxyl group at one end. After the MUA molecule is covalently bonded to the surface of the gold nanorod, it is then subjected to 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC). /N-hydroxysuccinimide (NHS) cross-linking agent, biomolecules can be easily bonded to the surface of gold nanorods, which is beneficial to the application of gold nanorods in biomedical fields. However, MUA is not water soluble, it is only soluble in organic solvents such as ethanol and chloroform, while gold nanorods are unstable in organic solvents, and direct modification with MUA leads to aggregation of gold nanorods. In 2007, a new method for completely replacing CTAB molecules on the surface of gold nanorods with MUA was reported. The ethanol solution in which MUA was dissolved was added to the gold nanorod solution, and ultrasonication was continuously performed to avoid aggregation of the gold nanorods, and MUA-modified gold nanorods were obtained. Although gold nanorods modified with MUA have been widely used in biomedical and other fields, aggregation of gold nanorods occurs during the experimental process, which requires strict control of experimental conditions to avoid aggregation.
Surface coating
The method refers to the inorganic nano material, the surfactant forming a single layer, a double layer or a multilayer structure on the surface of the gold nanorod by chemical bonding or physical adsorption, and the surfactant with a functional group can be anchored in the gold nanometer. The surface of the rod changes the surface properties of the gold nanorods. A simple modification method is to combine an anionic polyelectrolyte [such as sodium polystyrene sulfonate (PSS)] onto the surface of a gold nanorod coated with CTAB by electrostatic adsorption.
PSS not only converts the surface of the gold nanorods into a negative charge, but also allows the antibody to bind to the surface of the gold nanorods by hydrophobic interaction. In addition, polyacrylic acid (PAA) is also an anionic polyelectrolyte. Once PAA with carboxyl groups is modified onto the surface of gold nanorods, proteins can be covalently bonded to PANA-modified gold nanorods by EDC/NHS cross-linking. This is similar to the gold nanorod modified MUA. MURPHY et al. proposed a layer-by-layer assembly (LBL) method in which PSS and polydiallyldimethylammonium chloride were sequentially adsorbed on the surface of CTAB-stabilized gold nanorods by electrostatic action, and the process was repeated to get multilayer polyelectrolyte. This polyelectrolyte coating method is simple and fast, but the stability problem of polyelectrolyte coated gold nanorods still exists in long-term storage or in vivo experiments.
Another modification route is to surface coat gold nanorods using inorganic materials, and commonly used inorganic materials are silver, triiron trichloride, and silica. The coated composite is usually composed of a central gold nanorod and an outer layer of inorganic material. This composite structure not only prevents the aggregation of gold nanorods, but also covers a variety of biochemistry ligand. For example, silica has good biocompatibility, hydrophilicity, and chemical and colloidal stability, and is also susceptible to modification of surface biomolecules. Silica-coated gold nanorods provide an effective solution to the problem that surface biomolecules are difficult to modify. Gold nanorods modified by silica coating have been widely used in bioassay, biometrics and other fields.
Multi-level modification
Multi-level modification is a method of continuing to modify the surface of a gold nanorod that has been modified. Hyejin et al first attached the pyrithione to the surface of AuNRs, then treated it with sodium polystyrene sulfonate to make it negatively charged, and then modified the immunoglobulin to the surface of AuNRs by electrostatic interaction to make a functionalized Nanoprobes for highly sensitive specific labeling and imaging of cancer cell surface membranes.
At present, homogenous AuNRs with an aspect ratio of 3.5 have been synthesized by seed synthesis, and then the SiO2-coated AuNRs core-shell structure was successfully synthesized by sol-gel method. AuNRs@SiO2-NH2 and AuNRs@SiO2-FA were prepared by modifying amino and folic acid on the surface of AuNRs@SiO2, respectively. AuNRs@SiO2 is first treated by oxygen plasma purification. Next, the purified AuNRs@SiO2 was redispersed in anhydrous ethanol mixed with aminopropyltrimethoxysilane (APTS) for 24 hours. Then, the mixture was placed in an oven at 80 ° C for 2 h to maintain the stability of the function. The intermediate product GNRs@SiO2-NH2 was obtained after drying in a vacuum oven at 60°C for 3h. Next, GNRs@SiO2-NH2 was added to triethylamine (TEA) and n[p-maleimidopropyIoxy]succinimide ester (BMPS) for 30 min. After washing and drying, the substrate was added to folic acid cysteine for 2-3 hours, and modified to obtain AuNRs@SiO2-FA. The AuNRs@SiO2-FA sample was then collected by sonication and centrifugation.
Surface controlled modification of AuNRs-- two important routes.
AuNRs are commonly used as double-layer CTAB as a surfactant, which can be stably present in aqueous solution. The CTAB density at both ends of the gold nanorods is lower than that of the sides, allowing some molecules (such as thiols) to selectively replace the CTAB at both ends of the gold nanorods at low concentrations, and can also be selectively modified on the side of the gold nanorods by controlled conditions.
The ends and sides of the gold nanorods can be selectively modified by controlling the time and concentration of different ligand additions. At the beginning, the gold nanorods are stable in the aqueous solution and the CTAB bilayer. Since the radius of curvature of the gold nanorods is large at both ends, the CTAB bilayers connected at both ends are less than the end faces. Hydrolysis of tetraethyl orthosilicate (TEOS) forms a silicon coating.
In Route 1, since the silica coating is dynamically controllable, silicon can selectively coat both ends of the nanorod (as shown) because there are less CTAB at both ends of the gold nanorod, and silica coating The layer removes the CTAB molecules at both ends with very little energy. Continue to add metal salts, and then the metal will overgrow at the end faces, forming gold nanorods with silica modified at both ends.
In Route 2, the side of the gold nanorod is coated with a silicon dichloride coating. This method first modified mPEG-SH to the end of the gold nanorod using the method in Route 1, and the subsequent silicon coating was selectively modified on the side of the gold nanorod. In terms of preventing metal overgrowth, the silicon coating is stronger than mPEG-SH. Under the condition of continuing to add gold salt, gold is selectively deposited on both ends of the gold nanorod to form gold nanoparticles with end face modified with Silica.
These gold nanorods modified at both ends or in the middle with groups or polymers can also be assembled into ordered nanostructures through special interactions between molecules, such as end-to-end, side-by-side, which can be assembled. Into a more advanced structure, to achieve multi-dimensional assembly. In some respects, these compositions are superior in performance to individual gold nanorods, and their properties are the sum of the properties of all gold nanorods, exhibiting superior synergistic properties and expanding the use of gold nanorods.
Application of Gold Nanorods
Biological probe
At present, in the biological application of gold nanorods, firstly, some functional biomolecules such as antibodies and protein molecules are modified on the surface to form gold nanorod molecular probes, so that they have the function of specifically recognizing antigens. Since molecules such as antibodies are large and difficult to stabilize on the surface of gold nanorods, the usual solution is to first modify small molecules and then couple gold nanorods and antibodies through small molecules. The only drawback of this method is that gold nanorods are prone to aggregation in organic solvents. A large number of related studies have been conducted on this issue, and there have been successful cases in which MUA completely replaces CTAB without aggregation.
In 2007, IRUDAYARAJ et al. successfully coupled antibodies on MUA and specifically recognized target cells. In addition, another way to couple biomolecules to gold nanorods is to coat the gold nanorods with a polymer. The polymer is typically poly (sodium tetrastyrene sulfonate) (PSS) and poly (acrylamide hydrochloride) (PAH) which is capable of providing a carboxylic acid group on the surface of the gold nanorods. Therefore, a protein molecule such as an antibody can be attached to the surface of the gold nanorod under the action of an EDCINHS cross-linking agent.
At present, WANG et al first use Traut's reagent to attach an antibody to a thiol group. The antibody is not inactivated and specifically exists. The antibody-containing thiol can be directly bonded to the gold nanorods of different aspect ratios through Au-S on the surface. In this way, the problems encountered in the surface modification of the conventional gold nanorods can be eliminated, and the complicated process of biological functionalization of the gold nanorods is also simplified. In order to reduce the impact of non-specific binding, gold nanorods need to add a certain amount of polyethylene glycol thiol (PEG-SH) in order to improve the specific recognition of antigen. In summary, the thiolation of bioreceptors (antibodies) provides a new approach to the biofunctionalization of gold nanorods, which has made gold nanorods more promising for biomedical applications.
Biological sensing
AuNRs are very sensitive to the dielectric in the solution environment due to their unique surface plasmon resonance characteristics. Small changes in the electrolyte around the AuNRs cause local refractive index changes, which in turn lead to changes in the resonance frequency of the AuNRs plasma. Ultraviolet absorption spectroscopy is detected so that AuNRs can be used for biosensing using this property.
According to the data, breast cancer is still the most common cancer in women in developing and developed regions. A large number of early diagnosis and treatment methods for breast cancer are currently being studied, but no substantial progress has been made. The emergence of AuNRs has brought tremendous advances in breast cancer research.
AuNRs can be used in biosensors to detect human breast pain MCF-7 cells. Due to their unique chemical properties, AuNRs can easily adjust longitudinal plasmon resonance peaks in the visible to near-infrared range by controlling their morphology. Due to the presence of a near-infrared (NIR) window, AuNRs is considered a potential candidate for imaging or in vivo treatment, achieving deep penetration into soft tissue.
It has been reported that you currently have a MUC-1 aptamer-functionalized AuNRs that have been developed and applied to the detection of human breast cancer MCF-7 cells. This method has high sensitivity, good stability, simple operation and low cost. And this kind of cattle sensor may clarify the application of human breast cancer in early clinical diagnosis. It is believed that with the development of learning technology, this kind of cattle sensing technology may apply the main diagnosis and treatment of breast cancer.
Biological imaging
Due to its strong absorption signal, adjustable LSPR, and no light drift, AuNRs can be applied to multi-functional imaging technology to accurately predict and diagnose diseases. The LSPR effect can enhance the electric field on the surface of AuNRs, and the coupled electric field strength between AuNRs can obtain electromagnetic fields far larger than the incident electric field (such as surface enhanced fluorescence, surface enhanced absorption, surface enhanced Rayleigh scattering and SERS). Imaging and analytical detection can be performed by extracting an enhanced electromagnetic field signal.
Drug carrier
Drug controlled release system has become a research hotspot in pharmacy because of its low drug dosage, good therapeutic effect and small side effects. Today, nano drug-loading systems are increasingly favored by researchers. Studies have shown that the drug can be loaded onto the surface of AuNRs by physical adsorption or chemical bonding. The heat generated by the nanoparticles can change the surrounding environment and release the drug into the cell. When irradiated with near-infrared laser, AuNRs can produce surface plasmon resonance characteristics and photothermal effects, thereby achieving the purpose of remote light control carrying and releasing drugs.
Gene vector
Gene therapy can cure various diseases such as infectious diseases, genetic diseases, and cancer by making up for the defects of protein therapy. It is a new treatment method, and it is widely used in the therapeutic field, so it has attracted much attention. However, how to prepare safe and efficient gene vectors is a key element of gene therapy. Gene vectors are generally divided into two broad categories: viral and non-viral. Non-viral gene vectors are widely used because of their incomparable advantages such as low cytotoxicity and low immunogenicity, good biocompatibility, and easy biodegradation. Non-viral gene vectors include liposomes, cationic polymers, and nanoparticles. Gold nanorods have good application prospects in carrying DNA and SiRNA due to their large specific surface area, unique photoelectric properties, stable chemical properties, simple synthesis method, low toxicity and high yield. AuNRs can fully absorb infrared light energy and locally heat up, denature proteins and kill cells, thus showing special advantages in treating diseases.
Photothermal therapy
The near-infrared laser has the advantages of good penetrability and light damage to human tissues. The gold nanorods have good biocompatibility, low toxicity, good light stability, high photothermal conversion function, and can absorb wavelengths in the near-infrared region of 700-1000 nm. These advantages make the gold nanorods act as photothermal treatments. Good medium. In recent years, photothermal therapy of gold nanorods has been increasingly used in the treatment of tumors and infectious diseases. The experimental team has used chitosan modified gold nanorods to make biocompatible probes with good bioavailability, which provides a good prospect for in vivo targeted therapy. Another group has experimentally demonstrated that PEG-modified gold nanorods can perform photothermal therapy in vivo and ablate tumors in vivo.
Conclusions and Outlook
After nearly 30 years of development, the synthesis method of gold nanorods has been relatively mature, and its aspect ratio has been freely regulated. Moreover, gold nanorods have been successively applied in biomedical fields such as biosensing, cancer therapy and drug carriers, gene carriers and photothermal therapy. This paper reviews the synthesis methods, surface modification of gold nanorods and their application in biomedicine. The controllable modification of gold nanorods and their recent advances in biosensing, cancer therapy and drug carriers, gene carriers and photothermal therapy are highlighted. Although gold nanorods have had some initial applications in the biomedical field, there are still many problems that researchers need to continue to explore and research. For example, how to improve the selectivity or specific recognition ability of gold nanorods? How to solve the bottleneck problem of gold nanorods and inorganic nanocarriers in disease treatment? How to solve the problem of low fluorescence quantum yield of gold nanorods? How to accurately control the surface modification of gold nanorods? As well as the growth mechanism and asymmetric modification of AuNRs and their application in living organisms, are still unclear. These issues will become research hotspots in the next few years and even decades.
In response to the above problems, some experts believe that the introduction of molecular chiral or smart polymers into the surface of gold nanorods to prepare chiral gold nanorods or smart polymer modified gold nanorods may become a new idea. Chiral recognition is a very common and important recognition method between biomolecules. Therefore, more and more researchers introduce molecular chirality into the surface of nanomaterials to obtain chiral nanomaterials. We can also introduce chiral molecules. To the surface of gold nanorods to prepare chiral gold nanorods, this potential nanorod with potential chiral recognition will have a very broad application prospect in the field of drug carriers and bioprobes. On the other hand, smart polymers are a class of macromolecules that can respond to changes in factors such as force, heat, light, electricity, pH, and solvents in the environment, if such macromolecules can be modified to the surface of gold nanorods. Then, this kind of nanorod will combine the unique optical activity of gold and the intelligent response capability of smart polymer, which will have a very broad application prospect in the fields of environmental monitoring, sewage detection and atmospheric treatment.
References
[1] CHEN, Guo-zhen, WANG Jun, REN Hai-jing, LI Cong-ni, Research progress of gold nanorods application. Vol.43 No.1 Jan.2014
[2] Kelly K L, Coronado E,Zhao L L,et al. The optical properties of metal nanoparticles :The influence of size , shape ,and dielectric environment[ J]. J Phys Chem B ,2003 ,107(3) :668-677.
[3] Pan B F,Cui D X, Ozkan C, et al. DNA-Templated ordered array of gold nanoro in one and two dimensions[J]. J Phys Chem C ,2007 ,11(34):12572-12576.
[4]Orendorff C J, Hankins P L, Murphy C J. pH-Tiggered assembly of gold nanorods
[ J]. Langmuir. ,2005 ,21(5):2022 -2026.
[5] Rex M , Hernandez F E ,Campiglia A D. Pushing the limits of mercury sensors with gold nanorods[J]. Anal Chem,2006 ,78(2) :445451.
[6] Chen G Z,Jin Y, Wang W H, et al. Colorimetric sensing of Pb2+ using unmodified gold nanorods[ J ]. Gold Bull,2012, ,45(3) :137-143.
[7] Gou X C, Liu J ,Zhang H L. Monitoring human telomere DNA hybridization and G-quadruplex formation using goldnanorods[ J]. Anal Chim Acta ,2010 ,668(2) ;208-214.
[8] Xia Y S,Song L,Zhu C Q. Tum-on and near-infrared fluorescent sensing for 2 ,4 ,Chem ,2011 ,83(4) :1401-1407.
[9] Wang J,Zhang P,LiJ Y ,et al. Adenosine -aptamer recognition-induced assembly of gold nanorods and a highlysensitive plasmon resonancecoupling assay of adenosinein the brain of model SD rat [ J]. Analyst, 2010, 135(11) :2826-2831.
[10] HU Xue-Jiao GAO Guan-Bin ZHANG Ming-Xi.Gold Nanorods from Controlled
Synthesis and Modification to Nano Biological and Biomedical Applications. Acta Phys. Chim. Sin. 2017, 33(7), 13241337
[11] Cao, J,; Galbraith, E. K.; Sun, T.; Grattan, K. T. Sens. Actuators, B.2012, 169, 360. doi: 10.1016/j.snb.2012.05
[12] Ferlay, J.; Shin, H. R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D. W. Int. J. Cancer. 2010, 127, 2893. doi: 10.1002/ije.255 16.
[13] Stout, N. K.; Lee, S. J.; Schechter, C. B.; Kerlikowske, K; Alagoz,O.; Berry, D.; Buist, D.S. Cevik, M.; Chisholm, G.; de Koning, H.J.; Huang, H.; Hubbard, R. A.; Miglioretti, D. L.; Munsell, M. F.;Trentham-Dietz, A.; van Ravesteyn, N. T; Tosteson, A. N.;Mandelblatt, J. S. J. Natl. Cancer. Inst. 2014, 106, 092.doi: 10.1093/jnci/dju092.
[14] Bevers, T. B.; Anderson, B. O.; Bonaccio, E.; Buys, S.; Daly, M. B.; Dempsey, PJ.; Farrar, W. B.; Fleming, I.; Garber, J. E.; Harris, R.E.; Heerdt, A. S; Helvie, M.; Huff, J.G,; Khakpour, N.; Khan, S. A;Krontiras, H.; Lyman, G.; Rafferty, E.; Shaw, s.; Smith, M. L.;Tsangaris, T. N; Williams, C.; Yankeelov, T. J. Natl. Compr. Cancer Network 2009, 7, 1060.
[15]FU Jia-min, ZHANG Y u-juan, YUAN Keng , MIN Wei-pin.Functional Modification of Gold Nanorods and Its Application in Tumor Targeted Therapy. Journal of Nanchang University( Medical Sciences) 2017,Vol. 57 No.3
[16]XU Dongmei, LIUJian, GAO Jun, LIU Di, LIU Xiaowei .Gold nanorods:synthesi-s,properties,modification and applications.DOI: 10.1 6085/j.issn.1000-6613.2016.07.026.
© 2018 Robert Steven