Reason Why Ruby Is Red and Sapphire Yellow?
Ruby and Sapphire Both Consist of Aluminum Oxide
The crystalline form of Al2O3 (aluminum oxide) is called Corundum. It is found in many colors and shades, every shade may be named differently in different languages, in English deep red corundum (aluminum oxide) crystal is known as ruby and every other shade is known as sapphire, but both terms have a single name in Arabic that is Yaqut.
Why Aluminum Oxide Have So Many Colors?
If every crystal is made up of Al2O3 then from where did these different shades of color come?
Shades and colors arise when elements like iron, chromium, titanium, vanadium, copper, and magnesium replace few aluminum atoms in the crystal lattices of the crystal. Even though these are the atoms that shoot up the value of the stone, these elements in the crystal are called impurities by chemists!
-
Al2O3 with less than 0.01 percent of titanium in the crystal makes colorless sapphire.
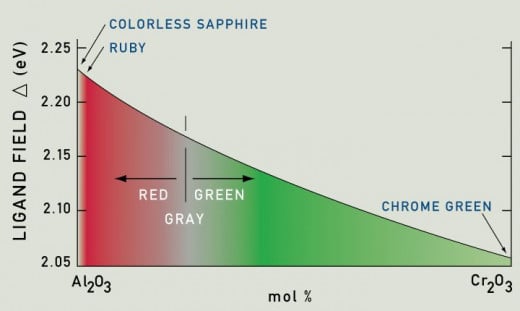
- Chromium atoms give the sapphire pink hue, more number of chromium atoms increase the redness of the crystal and it tends towards the formation of ruby, crystal with at least one percent o%f chromium is deep red-ruby. Pigeon blood ruby is the most sought-after color in rubies.
Chromium atoms absorb yellow-green light from white light resulting in red color stone. But don't assume that further addition of chromium atoms will increase redness. The graph below shows that further addition of chromium in the crystal will make it grey and green.
Pink sapphire and red ruby are different hues of the same crystal it makes it difficult to distinguish between them. Scientifically ruby should have more than 0.9% chromium ions in the crystal. Less than 0.5% will be counted as pink sapphire. 0.5% to 0.9% doesn't have clear definition.
- Trace amounts of vanadium in the crystal create purple shades in the sapphire.
- The most beautiful and expensive sapphire of blue hue and tones indicate the presence of only 0.01% iron and Titanium ions.
- If only iron atoms are present then stone is very pale yellow or green in color.
Shades of Sapphire

Shades of Yellow Sapphire (Yakut E Safra)
It Is Difficult To Draw a Line Between Ruby and Pink Sapphire

Color Chromium Gives to Corundum Crystals Proportional to Its Quantity In The Crystal
Is Green Sapphire and Emerald are Two Different Gems?
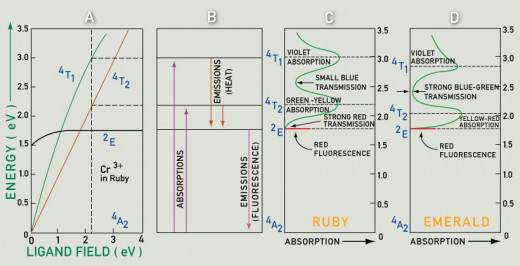
The above information is all related to ruby and sapphire which are known as Yakut in Arabic. Other precious stone known as emerald in English and Zamarrud in Arabic is green in color. The crystal of emerald is Beryl: beryllium aluminum silicate (Be2Al2Si6Ol8) and the green color is formed with the impurity of chromium. Now the amazing thing is that chromium as an impurity in colorless Corundum (Al2O3) makes red ruby and the same element in colorless beryl (Be2Al2Si6Ol8) makes out green emerald.
This difference of color by the same element is related to electrons' energy level and ligand field explained below in the graphs.
Graph 1