A Chemistry Guide: Explanation of the Basic Structure of the Atom
This is a very simplistic version of what happens at the atomic and subatomic level. This information satisfies high school chemistry standards and first year of chemistry in college standards.
Atomic Structure
The atom is the building block of all matter. Everything is composed of atoms in one state or another. However, there are even smaller particles that make an atom what it is. These are called subatomic particles. They are called this because they are smaller than an atom.
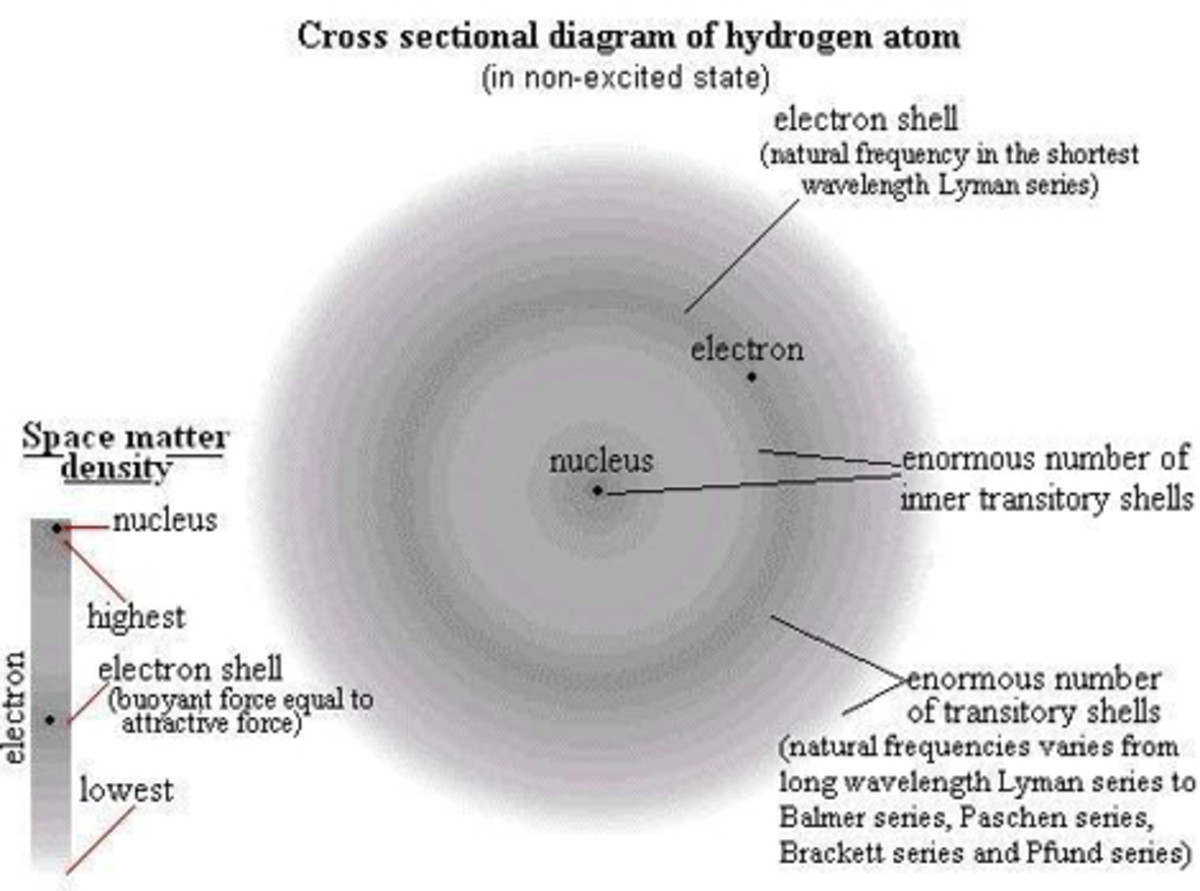
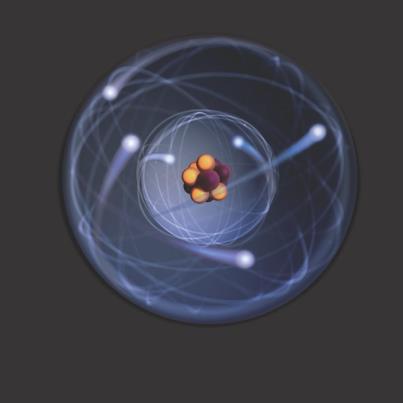
There are three subatomic particles that comprise an atom: protons, neutrons, and electrons. These are arranged within the structure of an atom. The two parts of an atom are the nucleus, which is the center, and the electron cloud, which is the surrounding area. Think about it like this: if you have a small rubber ball inside of a balloon filled with air, then the ball represents the nucleus and the air in the balloon represents the electron cloud.
Protons
Protons are one of two subatomic particles found in the nucleus. They have a positive (+1) charge. This means they attract things of the opposite charge. The number of protons found in the nucleus is called the atomic number. This number identifies the element on the periodic chart. The number of protons predicts the properties of the individual element.
Neutrons
Neutrons are the heavier subatomic particle (i.e. they have more mass). These are also found in the nucleus and have no charge (neutral charge). Neutrons determine the isotope of the element they are in. An isotope is a different version of an element based on atomic weight. Atomic weight is how heavy an atom is. To find atomic weight, add the number of protons and neutrons together. The average atomic weight of all isotopes is found on the periodic table.
Electrons
Electrons are the smallest subatomic particle in the atom. They have a mass so small that the number of electrons does not affect atomic weight significantly enough to be added (as far as basic chemistry goes). Electrons have a negative charge (-1). Therefore they are attracted to positive charges, such as protons. However, electrons also have extremely high kinetic energy. This means they move very fast. This is why they do not fall into the nucleus (as far as basic chemistry goes). They are located in the electron cloud. To help visualized how electrons move, think about tether ball. Looking at the tether ball setup from above, you see the pole, the ball, and the rope. Think of the pole as the nucleus and the ball as electrons. The ball gets hit and moves around the pole very fast. It cannot be inside of the pole though. The rope is the attractive force between the protons and the electrons which results from their opposite charges.
Orbitals
Every atom has orbitals that electrons inhabit. These correspond to an energy level. Electrons occupy the lowest energy level possible for them depending on how much kinetic energy they have. Electrons can move up and down between orbitals. This happens because an electron gets an energy boost from something such as light or heat. Once this energy is expended, the electron returns, or decays, to its original orbital. When an electron decays, it releases light or heat. Physical chemists explain in great detail how orbitals affect bonding, as well as structure and probabilities.
Bonding
Electrons are how atoms bond themselves to each other. As far as basic chemistry goes, they can do this in two ways: ionic or covalent bonding.
Ionic bonding results when an electron is pulled away from one atom to orbit another. This results in the two atoms having different charges. One will now have a slightly positive charge since it lost a negative electron. The other will have a slightly negative charge since it gained an electron. The opposite charges on these two atoms attract, which pulls them together.
Covalent bonding is where an electron is shared between two atoms, orbiting both and therefore binding them together. The electron revolves around one and then the other.