Determination of Sucrose Content in Fizz Drinks
Sugar is an important diet constituent which causes stress related ailments such as obesity and diabetes. Stress which is the curse of modernization, lowers the glycogen content of the body. It makes a consumer reach out for a sugary drink. Sugar absorption into the cells is regulated by the hormone insulin. When there is rise in blood sugar levels, immediately insulin is produced. Insulin is the only hormone that has to counteract all other hormones which tend to increase the blood glucose levels. Most of these counteracting hormones are released during stress. Mental distress or overwork increases the body's demand for sugar. Sugar gives energy to our muscles and to the brain. Therefore, to a knowledgeable consumer, the label claim of the product becomes an important feature in choosing a product.
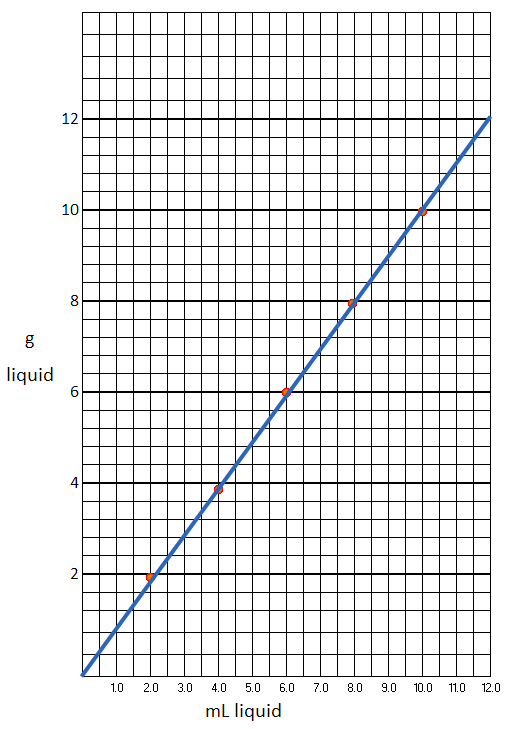

Requirements-* standard volumetric flasks, electronic balance,weighing paper, spatulas, distilled water, sucrose,etc
In order to calculate the molarity and molality of a given mass % sugar solution.The exact amount of sugar was taken as calculated and weighed accurately. It was dissolved in a beaker. containing 100 mL of the solvent and added to a pre-weighed beaker. We now have the mass and volume of the solution. Thus, we can calculate the molality and molarity of the solution, as also the density of the solution!
We repeat the procedure of determining the mass and volume of the fizz drink. Exactly 100 ml of the solution of commercial sodas were taken and weighed. The densities of the fizz drinks devoid of the fizz in the solutions, were calculated [ D=M/V]; The corresponding densities for unknowns from the standard curve of the sugar solution were determined. Note the mass% of sugar in the solution. The results were verified from the value given on the label of the drink.
1. Mass% - :it is defined as the number of parts by mass of the solute per hundred parts by mass of the solution
Mass percent of solute=[(weight of solute)/(weight of solution)]*100
2. Molarity -it is defined as the number of moles of the solute in 1L of the solution.
Molarity = [(moles of solute)/Volume of solution)].
3. Molality -it is defined as the number of moles of the solute in 1kg of the solution.
Molality = [(moles of solute)/(mass of solution)].
Thus, determination and confirmation of the sugar content in any drink can be done by a simple density determination.