- HubPages»
- Education and Science»
- History & Archaeology»
- Major Inventions & Discoveries
Fritz Haber: In Peace and War

It has been said that half of the current world population owed their gratitude to one brilliant German chemist, Fritz Haber. Among his numerous contributions to science, Haber's most prized discovery has made it possible to feed the constantly growing population during a time when everyone was worried that humanity might have exceeded earth's ability to produce food. However, Haber also personifies science's capacity to serve causes on both extreme ends of good and evil. In answering the call of duty, Haber marched into the battlefield of World War 1, applying his gift of ingenuity into a terrible instrument of war that would claim the lives of his country's enemies.
A Humble Beginning
The son of first cousins Siegfried Haber and Paula Haber, Fritz Haber was born on December 9, 1868 in Breslau, Germany, which is now in current-day Poland. His mother died shortly after giving birth. His father was a well known chemical merchant. After his father remarried, he was blessed with 3 half-sisters whom he had a pleasant relationships with, unlike the difficult relationship he had with his own father. Throughout much of Christian Europe, the Jews were victimized because of their main profession as moneylenders and the rooted differences in Christian and Jewish beliefs. Fortunately for Haber, a Jewish rights movement in the 19th century achieved success in their effort to be granted equal treatment and rights as citizens of German. Under the edict, members of Haber family and other Jews were able to establish positions for themselves in business, academics, politics and law. His family continued to support the Jewish community and traditions although Haber identified himself more as a German citizen than a Jewish.
Educations
- St. Elizabeth Classic School, Breslau, Germany
- Friedrich Wilhelm University, Berlin, Germany
- University of Heidelberg, Heidelberg, Germany
- Technical College of Charlottenburg, Berlin, Germany
- Frederick William University, Berlin, Germany - Haber was awarded with PhD in 1891
Career
- Institute of Technology, Zurich, Switzerland
- University of Jena, Jena, Germany
- Karlsruhe Technische Hochschule, Karlsruhe, Germany - Haber was conferred full professorship in physical chemistry in 1906

The Threat of Malthusian Catastrophe
By the early 1900, European nations enjoyed rapid development in the Industrial Age. The increased in life expectancy was accompanied with a steady growth in population. Almost a century earlier, an English economics scholar, Thomas Robert Malthus authored a book that painted a grim future brought on by industrial development. In his book 'An Essay on the Principle of Population', Malthus argued that while population will always multiplies geometrically, food production can only grow arithmetically. In simple terms, there will come a time when the rate of population growth will exceed earth's ability to produce subsistence for mankind. In order to counter the imbalance, Malthus proposed two countermeasures to return the population within resource limits; preventive checks, which are meant to lower the birth rate such as birth control, abortion and celibacy. The other one, which hold the devil's details, are meant to increase the death rate such as plague, war and famine.
With civilization on the brink of extinction, scientists across Europe began the race in the year 1898 to find a solution that will negate Malthus's prophecy. In particular, scientists committed their efforts to find a way to increase crop yield by researching an economical method to synthesize ammonia, an essential ingredient in the manufacture of fertilizers. The ability to synthesize ammonia was highly sought after because it is a compound that can provide rich nitrogen supply and encourage crops to produce more food. Before Haber's discovery, there were only 2 ways to deliver source of nitrogen to crops. The first was through manure and human waste, which was obviously inadequate for large-scale farming. The second method was to import South American guano and sodium nitrate deposits; both proven to be excellent sources of nitrogen. However, in 1900, the South American deposits diminished rapidly and the cost of the deposits rose due to lack of supply in times of high demand. It became clearer to industrialists and scientists that the only viable option for survival was to fabricate ammonia from an untapped source of nitrogen; the atmosphere, which was known at that time that the air contains up to 80% of nitrogen. Additionally, converting nitrogen from the air into usable form such as liquid is called 'nitrogen fixation'.
Haber's Groundbreaking Discovery
Haber's quest in finding the optimum method for nitrogen fixation began in 1905 after he finished writing his third textbook, 'Thermodynamics of Technical Gas Reactions'. Haber built upon and improvised his nitrogen fixation technique based on previous works by Henry Le Chatelier, Kristian Birkeland, Samuel Eyde, Walther Nernst, Nikodem Caro and Adolph Frank, whose works required the application of temperature as high as one thousand degrees centigrade, which was not practical for industrial-scale production. Chatelier's method, which necessitates the utilization of high pressure, proved to be risky after one of his assistants was killed from an explosion of a sealed container that can't withstand high pressure.
Together with his assistant Robert Le Rossignol, Haber experimented with various temperature, pressure and metal catalyst. The utilization of metal catalyst, in a way contributed to his success as it played a crucial role in breaking apart the otherwise stable atmospheric nitrogen molecule. To further intensify the yield and save energy, Haber redirected the heat from the reaction back to itself to provide a continuing cycle of energy necessary for chain reaction. Finally, their endeavor came to fruition in the summer of 1909 when they finally succeeded in producing ammonia from air, drop by drop, at the rate of about 125 ml per hour.
To convert his laboratory-scale ammonia synthesizing process to industrial scale, Haber collaborated with Karl Bosch, whose company, BASF had been supporting Haber's research. Bosch took the challenge of scaling up the production of ammonia to meet industrial's needs and succeeded in 1910. The manufacture of commercial quantities of ammonia was finally made possible through the hard work of Haber, Rossignol and Bosch. With endless supply of ammonia churning out from factories, huge amounts of fertilizers was produced which brings an era of significant increase in crop yields and thus banishing the risk of famine in large parts of the world. Some called the Haber-Bosch process nothing short of a miracle, akin to creating 'bread from air'. Until today, the Haber-Bosch process is still considered a gold standard in the mass production of ammonia which leads to more than 100 million tons in current annual world production of synthetic nitrogen fertilizer. The magnitude of Haber's achievement is best described by Vaclav Smil, who authored the 2001 book 'Enriching the Earth: Fritz Haber, Karl Bosch and the Transformation of World Food Production'.
The industrial synthesis of ammonia from nitrogen and hydrogen has been of greater fundamental importance to the modern world than the invention of the airplane, nuclear energy, space flight, or television. The expansion of the world's population from 1.6 billion people in 1900 to today's six billion would not have been possible without the synthesis of ammonia.
— Vaclav Smil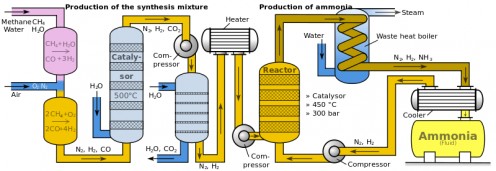
An Opportunity in Times of Darkness
After his success in the mass production of ammonia, Haber was appointed director at Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry in Berlin, Germany. However, the beginning of World War 1 in 1914 would present an opportunity to Haber; to prove his undivided allegiance to his fatherland Germany. All his life, Haber had done all he could to present himself as a patriot and severing the ties of his Jewish heritage. He converted from Judaism to Lutheranism. He assimilated in German culture and adopted it as his own. The German state, Kaiserreich, was his state. He grew up making a name for himself among scientists and industrialists in a time when Germany prided itself as a powerhouse in the Industrial Age. To his friend Rudolf Stern, he uttered the following phrase;
I considered myself a 100 percent German and under the impact of philosophy and science, of the whole rational temper of the word, no longer felt any ties to the Jewish religion.
— Fritz HaberThe Rise of Doctor Death
Haber was one of the 93 Germany intellectuals who signed the 'Manifesto of the Ninety-Three' in October 1914. The manifesto was a declaration of unequivocal support from scholars for Germany's military efforts in World War 1. Haber turned Kaiser Wilhelm Institute into a coordination center for the war's research and development projects. He managed to attract more than 1,300 technicians and 150 scientists to the Institute. Among the famous names that collaborated with Haber in Germany's war efforts were Otto Hahn, James Franck and Gustav Hertz. During his service in the war, he developed alternatives to traditional antifreeze to keep vehicles running and his ammonia synthesis methods were used to create nitric acid, a key component in explosives and ammunition. But the ingenuity of Haber in the creation of weaponries didn't stop there. Ongoing works at the center also was dedicated to the research and application of chemical warfare in the battlefield.
First Chemical Warfare
Haber argued that in order to bring the war to a quick conclusion, it was simply not enough to adhere to the common tactics of warfare, such as increased firepower and sending more troops to the front lines. What was needed back then was an unconventional means to flush the enemies from their trenches and resume open warfare. In his thinking, although any type of irritant gas would work, it is better to add more elements of surprise and terror in the gas employed. Haber expected a shock reaction to be brought about by true chemical weapons, not just incapacitating but lethal gases, all-pervading, unstoppable, and leaving a free way for advancing German troops. Thus, the first iteration of chlorine gas (sometimes called bertholite) for warfare materialized under Haber's supervision. On April 22 until 25 May, 1915, Haber himself marched into the Second Battle of Ypres in Belgium with the German Army, eager to supervise and witness first-hand the consequences of the deployed chlorine gas on the Allied troops.
The method to release the chlorine gas was very simplistic in nature. Theodore Gray wrote in his book 'The Elements: A Visual Exploration of Every Atom in the Universe', "Soldiers would position a line of gas cylinders at the front lines, wait for the wind to shift towards the enemy, then open the valves and run like hell." And since chlorine gas is denser than air, it would quickly blanket the enemy troops in the trenches and effectively cutting off their oxygen supply, forcing the troops out in the open and vulnerable to line of fire from German troops. And that was not the worst part of it all. Even if the Allied troops managed to fight the instinct and remained in the safety of the trenches, they were just mere minutes away from suffocating to death. Hydrocloric acid can be formed inside their lungs due to the reaction between the inhaled chlorine gas and the mucosa of the lungs. It was an agonizing death, being poisoned by the very air that the troops breathed in and simultaneously going through to the torture of losing eyesight and stung sensations at the back of the throat and chest.
The Aftermath of War and Haber's Legacy
Marriage
Haber's participation in the campaign at Ypres resulted in 67,000 deaths of Allied troops. All those while, Haber was fighting another battle that he was destined to lose; the quarrel with his wife, Clara Immerwahr. Clara, like Haber, was also a talented chemist. She was the first woman to received PhD from University of Breslau and a women's rights activist. She was also a pacifist, from which the same ground she denounced her husband's role in the war. Clara was particularly horrified at the unconventional tactics employed by Haber in the battlefield. Being a chemist herself, she understood well enough the effects of poison gas on human biology. The quarrels between them finally reached the climax on 2 May 1915, when Clara committed suicide in their home by using Haber's revolver to shot herself in the chest. She was attended immediately by their 13-year old son, Hermann, although it was too late for Clara. Ironically, this tragedy took place while Haber was reportedly celebrating his promotion to Captain in the military rank. After a few days of mourning, Haber once again left behind the only family he had, his son, on another duty at the Eastern Front to supervise another series of chemical warfare against the Russian army. In October 1917, Haber tied the knot for the second time to Charlotte Nathan and they had 2 children. Unfortunately for Haber, he divorced his second wife 10 years later due to marriage conflicts.
Nobel Prize
Fritz Haber was awarded the Nobel Prize in Chemistry in 1918, although he only accepted the award after the war concluded in November 1919. He was honored with the award on the ground of his works on the Haber-Bosch process and its immediate benefit to the survival of mankind.
Gold from the Ocean
As a display of patriotism, Haber conducted experiments to extract gold from seawater. Haber was hoping that gold obtained by this method would eased the burden on Germany's responsibility to pay off debts. However, the effort proved to be unsuccessful as the cost required to extract the traces of gold exceeded the value of gold itself. The only consolation from this effort was that Haber found a precise method to measure the concentration of gold in liquids.
Haber Colloquium
After his shortfall in the gold extraction project, Haber redirected his efforts in reorganization of the Kaiser Wilhelm Institute, to which he appointed sectional directors with complete freedom in their work. Through the institute, he organized the Haber Colloquium, a fortnightly seminar after the war that brought together exceptional talents in physics and chemistry, such as Peter Debye, Nielhs Bohr and Otto Warburg. His reputation as an outstanding scientist earned him respects from the international scientific circle who were fortunate enough to attend his colloquium. In 1953, the Kaiser Wilhelm Institute was renamed Fritz Haber Institute in his honor.

Haber's Exile and Final Moments
In 1933, the rise of National Socialism coupled with Adolf Hitler's ascension as the Chancellor of Germany presented a great disturbance to Haber's career. Even with his status as a decorated war veteran, German patriot and Protestant Christian didn't guarantee he will be spared from discrimination, as the National Socialism moved with full intent to purge Jews out from Germany. With a heavy heart, Haber tendered his resignation as the Director of Kaiser Wilhelm Institute and professor at the university on 1 October 1933. Haber moved to England and worked at Cambridge under the invitation of his friend, William J. Pope. He lasted there for 4 months before accepting an offer as the director in the physical chemistry department of Daniel Sieff Research Institute, Rehovot, which is now in current-day Israel. However, on 29 January 1934 Haber succumbed to heart failure during the journey to his destination. Haber's eternal resting place can be found at Hörnli Cemetery, Basel, Switzerland where his grave is situated next to the grave of hist first companion, Clara Immerwahr.