How to Calculate Effective Nuclear Charge
Effective Nuclear Charge
Effective nuclear charge is the shielding effect of lower level electrons protecting valence electrons from the pull of the nucleus. The nucleus acts as a magnet to pull in electrons, but not all of them feel the full force of this magnetic attraction.
In short the lower the number, the less the electron in question feels the force of the nucleus pulling on it making it easier to be taken.
The formula is as follows:
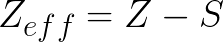
Effective Nuclear Charge Practice
Use the formula to get the effective nuclear charge of Na and Br. Then compare the charges to see which one is more likely to give up electrons mathematically!
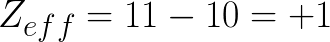
1. Na
First let's find out the atomic number of Na, it's 11, and use that as our Z. Next let's see how many electrons are in the valence shell. Since it's the first element on the 3rd row we know that has 1 in it's valence shell with 11 total. So we figure out how many of those electrons are NOT in the valence shell 1-11=10, so we plug in 10 as S and we solve!
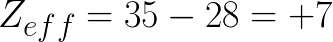
2. Br
First we will find out the atomic number of Br, it's 35, this is Z. Next we find the number of electrons that are NOT in the valence shell. Based on it's position in the table we can count from the left to the right in the 4th row (making sure to skip the metals) we see it's the seventh element meaning it has 7 valence electrons so 35-7=28 so we use this as S.
Effective Nuclear Charge Conclusion
We can conclude then that based off of mathematically comparing Na (Zeff = +1) to Br (Zeff = +7) that Na's valence electron feels less positive force than Br's valence electrons.
Element
| Atomic Number
| Valence Electrons
| Zeff
|
|---|---|---|---|
Li
| 3
| 1
| +1
|
C
| 6
| 4
| +4
|
Si
| 14
| 4
| +4
|
Ca
| 20
| 2
| +2
|
Sr
| 38
| 2
| +2
|
A Few Effective Nuclear Charges
Consider the following table to ensure you understand how to calculate them without error. You will notice a pattern. Once you notice the pattern you will start to see yet another reason why the periodic table of elements is so ingeniously organized!





