Rare Earths - Chemistry and Applications
Introduction
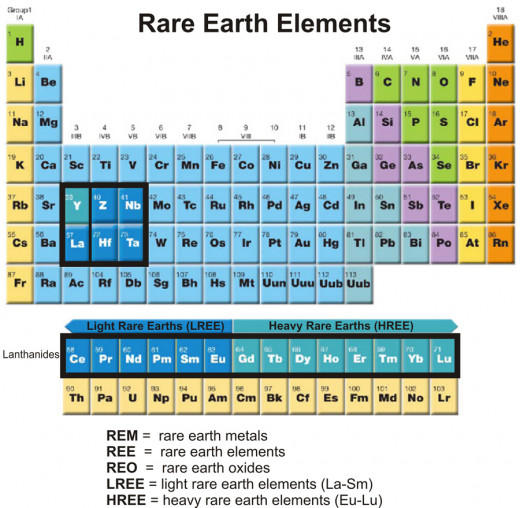
The term “rare earths” refers to a group of fifteen elements of the periodic table - lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). These elements have atomic numbers ranging from 57 (La) through 71(Lu). They are placed in group (vertical column) III-B and period (horizontal row) 6 of the periodic table. Since there is only one spot in the periodic table for all these elements in the series, they are listed separately at the bottom of the table. It was believed that these elements are “rare” in nature and are found in the form of “earth” (oxide). Hence, the term “rare earths” was coined. However, with the passage of time and advancement of knowledge it is revealed that these elements are not as “rare” as were once thought and also not available in the form “earth” (oxide) in nature. These elements are now termed as “lanthanide group elements”, as lanthanum is the first element in the series. All elements in this series have similar chemical properties. Elements scandium (Sc, atomic number 21) and yttrium (Y, atomic number 39) of group III-B have almost identical physical and chemical properties to that of lanthanide group elements. Hence, they are also included in the lanthanide group. However, the traditional term “rare earths” is still in use. All the rare earths except promethium (Pm) are available in nature. Promethium is obtained by thermal neutron fission of Uranium (U235) [1]. It is also obtained by bombarding neodymium with neutrons produced from uranium fission in a nuclear reactor [1].
History
The story of rare earths spans a period of 160 years. The history of rare earths is very interesting. The first rare earth mineral ytterbite (yttria) was discovered in 1787 by Carl Axel Arrhenius [2]. The last addition to the rare earth group was the element promethium which was artificially produced in 1947 by Jacob Marinsky, Lawrence Glendenin, and Charles Coryell [1]. Many other inventions were made during this period which contributed to the discovery of rare earths. The most significant contributions are the development of the periodic table by Dmitri Ivanovich Mendeleev [3] and Lothar Meyer, the discovery of X-rays by Wilhelm Conrad Roentgen [4], the invention of atomic numbers by Moseley [5], discovery of atomic structure by Neils Bohr [6] and Ernest Rutherford [7], the discovery of emission spectroscopy by Gustav Robert Kirchhoff and Robert Wilhelm Bunsen [8], and work on the atomic bomb during World War II [1].
In 1787, Arrhenius, a Swedish army officer, found an unusually heavy black rock in a stone quarry in a village called Ytterby, close to Stockholm, Sweden. In 1794, a Finnish chemist, Johan Gadolin analyzed this mineral - later called gadolinite - and found a hitherto unknown earth called ytterbia whose name was later shortened to yttria [9]. In 1903, Swedish chemist Jons Jacob Berzelius and minerologist Wilhelm von Hisinger discovered the earth ceria [10].
The discoveries of the so called minerals yttria and ceria were the starting point for the studies of rare earth elements. The separation of the different components of these minerals was a difficult task, since they are chemically very similar to one another. Over the course of a century, yttria and ceria were separated into eight and seven stable elements, respectively [11]. Of the rare-earth metals, many - terbium, erbium, ytterbium, scandium, thulium, holmium- have been given names that show their origin in various Swedish localities. Swedish chemists, chief among them being Mosander and Cleve [12], have made valuable contributions in this domain of chemistry.
Chemistry of Rare Earths
The chemical properties of metals are determined by the valence electrons. Generally, these are the electrons in the outermost shell of the atom. These are responsible for the broadly similar chemical properties of all lanthanide group elements. The finer distinction in the chemical properties of the rare earth elements, is determined by the electronic configuration of their trivalent ions, in particular, by the number of electrons in its 4f sub-shell. The electronic configuration of the rare earth ions is [Xe] 4fn, where [Xe] refers to the electronic configuration of the element xenon and n represents the number of electrons in the 4f sub-shell. In rare earths, 4f sub-shell is shielded by the 5s2 and 5p6 closed sub-shells. Within the rare earth series, the electrons are added to the 4f sub-shell and not to the outermost, sixth, shell. Therefore, the exterior parts of the atomic structure throughout the series remain virtually unchanged. As a result, their chemical properties are very similar. Lanthanum, (4f0 is the first element and lutetium (4f14) is the last element in the series.
Contrary to the general trend in the periodic table, it has been observed in the rare earth series that the atomic radii of the elements and their ions decrease slightly, as the atomic numbers increase (starting with Ce). Between one element and the next in the rare earth series, an electron is added to an inner incomplete 4f sub-shell, which is shielded by the outer 5s2 and 5p6 sub-shells. This effect of this shielding is that the electron is pulled closer to the nucleus and hence, the radius of the subsequent element and its ion is slightly smaller. This behavior is known as the lanthanide contraction. The difference in ionic radius of the adjacent rare earths is very small. The ionic radius of Ce+3 is 1.06 Å and that of Lu+3 is 0.85 Å [13].
Separation of Rare Earths
It is stated earlier that from the minerals yttria and ceria, eight and seven rare earths, respectively, were separated over a period of 100 years. The development of separation techniques took such a long time because the chemical behavior of all the rare earths is very similar. The separation of adjacent rare earths is difficult because the difference in ionic radii of the adjacent rare earths and the difference in their ionization potentials (electron binding energy) is very small [14].
All the methods of separation of individual trivalent rare earth ions are based on the difference in their basicity. Due to both the above (interrelated) reasons, the difference in basicity of the adjacent rare earths is very small [15]. The basicity of the rare earths affects the solubility and stability of their salts and complexes [16]. Therefore, the difference in solubility and the difference in stability of the salts and complexes of adjacent rare earths is very small. These are the key reasons why it is difficult to separate the salts and complexes of these rare earths from each other.
To increase the difference in stability of the complexes of the adjacent rare earths, chelating agents are used. A chelate is defined as a combination of a metal ion with an organic molecule (chelating agent) to form a ring like structure. Chelates are special type of complexes which are much stronger than ordinary complexes. The difference in the stability of the chelates of two adjacent rare earths is evidently more than the difference in the stability of ordinary complexes of the same rare earths. This principle is used to overcome, to a certain extent, the difficulties arising in separation as a result of very similar basicity of the adjacent rare earth ions.
Based on this principle, methods such as fractional crystallization, fractional precipitation, ion-exchange (solid ion exchangers) and solvent extraction (liquid ion exchangers) were developed for the fractionation of rare earths and separation of individual rare earths. C. K. Gupta and N. Krishnamurthy have summarized all the methods of separation of rare earths [15].
FRACTIONAL CRYSTALLIZATION
In fractional crystallization a part of the salt in solution is precipitated by a change in temperature or evaporation of a saturated solution. If the solubilities of the different metal salts in the solution differ, the composition of the precipitated crystals will be different from that of the original solution. While the crystals of the less soluble component get enriched, the more soluble components simultaneously get enriched in the solution. Fractional crystallization is very slow and tedious. In early 1900s Charles James used rare earth bromates and double magnesium nitrates for fractional crystallization of rare earths. This is known as the "James Method” [11]. Enormous number of stages of crystallization were required to get the high purity of individual rare earths. For example, rare earth bromates had to be crystallized for four years daily to obtain good quality holmium and 15,000 times to get good quality thulium (it still contained some ytterbium and lutetium [15]. His method was used widely and considered to be the best for separating rare earth elements until the discovery of ion exchange in the 1940s. Letters in the University of New Hampshire archives show that James supplied his rare earth compounds worldwide. The American Chemical Society recognized his work as a National Historic Chemical Landmark at the University of New Hampshire on October 29, 1999. The plaque commemorating the event reads [11]:
“Beginning in 1906, in a laboratory in Conant Hall, Charles James (1880-1928) devised novel fractional crystallization techniques for separating rare earth elements, which were widely adopted by other chemists. James used his method to separate large amounts of ytterbium, hitherto considered to be a single element, into two elements now known as ytterbium and lutetium. When the simultaneous isolation of lutetium was published in 1907 by Georges Urbain, James made no public claim for his own pioneering work. Despite his retiring nature, James was internationally recognized as an expert in rare earth chemistry. His highly purified rare earth specimens were in demand by research laboratories throughout the world”.
FRACTIONAL PRECIPITATION
Fractional precipitation involves the removal of a fraction of the rare earths from solution by addition of a chemical reagent that forms a less soluble compound with some of the rare earths. The rare earths still remaining in solution can be recovered by further precipitation. The double sulfates RE2(SO4)3 Na2SO4 nH2O (RE stands for rare earth) are usually precipitated by the addition of a solution of Na2SO4. The rare earths, La, Ce, Pr, Nd, and Sm form sparingly soluble double sulfates whereas Ho, Er, Tm, Yb, Lu, and Y form soluble double sulfates and Eu, Gd, and Dy form double sulfates of intermediate solubility. Generally the use of this method is confined to crude separation of the rare earths mixture into three groups [17].
ION-EXCHANGE
Ion-exchange technique for the separation of rare earths is based on the differential absorption and, mainly, differential elution of ionic species, both of which are affected by the basicity of the rare earths. To increase the difference in basicity of the adjacent rare earths, chelating agents such as ethylene di-amine tetra-acetic acid (EDTA), nitrilo tri-acetic acid (NTA), citric acid, etc. are used [18]. This achieves better separation of individual rare earths. It is pertinent to note that the stabilities of the chelates invariably increase from La+3 to Eu+3 or Gd+3. But beyond Gd+3, the stabilities may continue to increase from Gd+3 to Lu+3, or may remain nearly constant, or pass through a maximum called “gadolinium break [19]. Ion-exchange technique was first used for the separation of rare-earths during World War II to separate fission products obtained from nuclear reactors [20]. Since then it was widely used because of its simplicity and ability to produce high purity material. However, the process was very slow and not continuous. Since 1970s, solvent extraction processes are available for the separation of rare earths and hence, ion-exchange technique is now almost outdated.
SOLVENT EXTRACTION
Solvent extraction technique is used to fractionate rare earths and also to separate individual rare earths. This technique is based on the principle of relative solubilities of metal ions in two different immiscible solvents usually water and an organic solvent, such as kerosene, containing an extractant which has the ability to extract the metal in the form of a complex. A cation-exchanger such as di-2ethylhexyl phosphoric acid, an anion exchanger such as alamine-336, or a solvating type extractant such as tri-n-butyl phosphate can be used as extractants. As the difference in basicity of adjacent rare earths is small, the separation factor, in particular, between the two adjacent rare earths is very small. Hence, enormous number of stages of extraction are required to get the desired purity and separation of adjacent rare earths. Solvent extraction processes are very fast and continuous and, hence, are widely used. Ritcey and Ashbrook [21]have lucidly described the basic principles of solvent extraction of metals and its application to process metallurgy.
SEPARATION BASED ON SELECTIVE OXIDATION-REDUCTION
The methods of separation described so far are based on the difference in basicity of trivalent rare earth ions. Though all the rare earths form RE+3 ions, some of them also form RE+2and RE+4 ions, where RE refers to a rare earth element. It is well known that 4f0 (empty sub-shell), 4f7 (half filled sub-shell) and 4f14 (completely filled sub-shell) are the most stable configurations and these are responsible for the existence of RE+2 and RE+4 oxidation states. The ion Ce+3(4f1) attains the Ce+4 state and the ion Tb+3(4f8) attains Tb+4 state by losing an electron from the 4f sub-shell and arriving at the stable configurations of 4f0 and 4f7 sub-shell respectively. Likewise the ion Eu+3 (f6) attains Eu+2 state and the ion Yb+3 (f13) attains Yb+2 state by gaining an electron and arriving at the stable configurations 4f7 and 4f14, respectively. In the rare earth series, Pr is adjacent to Ce and exhibits tetravalent state like Ce. Similarly Sm is adjacent to Eu and exhibits divalent state like Eu.
Selective oxidation of Ce, Pr, and Tb, and selective reduction of Sm, Eu, and Yb are useful in effective separation of these elements from the other trivalent rare earths because these oxidized (RE+4) and reduced (RE+2) states, exhibit markedly different chemical behavior compared to the trivalent (RE+3) state of these rare earths. Apart from usual reduction methods, Eu can be separated from other trivalent rare earths by photochemical reduction followed by precipitation as EuSO4.
Salts of individual rare earths obtained by any method are eventually converted to metals. Techniques, such as mercury amalgamate oxidation-reduction, vacuum distillation, and metallothermic reduction have been used for the preparation of rare earth metals. Ultra high purity metals Eu, Gd, and Lu with less than 10 ppm. total metallic impurities have been made using these techniques [22].
Sometimes, claims of high purity (such as 4N purity) metals do not take into account the interstitial elements present in the metal. In fact, they are not removed during refining. In some instances, the metals formed contain around 1% oxygen. This is enough to form a considerable volume of internal oxide which is likely to make the metal highly brittle. Due to such interstitial impurities high-tech applications of the rare earth metals are likely to be hampered [23].
Characteristics of Absorption Spectra
This section discusses the spectral properties of rare earths which have varied applications. Absorption bands produced by trivalent rare earth ions in the visible region originate in the 4f energy level. The energy states of the electrons that produce the absorption bands are largely due to the transitions between two energy states of the same 4f configuration [24,25]. These are instances of forbidden transitions in which the electron undergoes a change in energy state without leaving the 4f sub-shell. (In spectroscopy, forbidden transitions are defined as those having very low probability due to the violation of certain selection rules). Rare earth ions have intrinsically low extinction coefficients due to intra-4f transitions. The absorption spectra exhibited by compounds of rare earths in crystalline state are more complex than would be expected theoretically from the fundamental transitions involved. The additional line-like bands that appear in the spectra are ascribed to the splitting of the original bands by the electrical field of the crystal, the degree of splitting being determined in part by the symmetry of the crystal structure [25], and in part by the nature of the other ions with which the absorbing ion is associated [26]. The absorption spectra produced by rare-earth ions in crystalline substances, therefore, differ somewhat from those produced by the same ions in aqueous solutions, but the general distribution pattern of the absorption bands still remains essentially the same.
Magnetism in Rare Earths
The magnetic properties of materials are, in general, determined by the nature and magnitude of the atomic magnetic moments. Bohr magneton is the fundamental unit for magnetic dipole moments.
Paramagnetism is the tendency of the atomic magnetic dipoles to align with an external magnetic field and arises due to unpaired electrons. Except La (4f0) and Lu (4f14) all the rare earths in the series contain unpaired electrons and are, therefore, paramagnetic. The magnetic dipole moment of the paramagnetic material is due to the orbital motion of the unpaired electrons around the nucleus and the spinning of the electrons and it varies with temperature. Paramagnetic behavior can also be observed in magnetic materials that are above their Curie temperature, the temperature at which ferromagnetic property of all ferromagnets disappears as a result of thermal agitation. In principle, the chemical environment changes the magnetic moment of an atom. However, in the case of rare earths, on complexation, there is no significant change in magnetic moments. This is an indication that 4f electrons do not take part in a major way, in bond formation.
In contrast to paramagnetism, diamagnetism is the tendency of all materials to oppose applied magnetic fields and arises due to paired electrons. It can only be observed in materials that do not exhibit any other forms of magnetism. The rare earths La and Lu are diamagnetic as they contain only paired electrons. There is also hidden diamagnetism in paramagnetic rare earths due to paired electrons in other shells.
All theoretical aspects concerning the magnetic properties of rare earths have been described in detail by J. Jensen and A. Mackintosh [27].
Applications
Numerous applications of rare earths have been summarized by J. B. Calvert [28] , and C. K. Gupta and N. Krishnamurthy [15]. Some of the applications from these reports are reproduced here.
The largest use of rare earths has been in glass polishing and ceramics, 39%, followed closely by automotive catalytic converters, 22%. Permanent magnets account for 16%, petroleum refining catalysts 12%, metallurgical applications 9%, cathode-ray tube phosphors 1%, and miscellaneous uses, including lighter flints, account for 1% of rare earths consumption.
In 1891 Carl Auer von Welsbash produced a gas mantle. This is the first application of rare earths. In this process a mixture of 99% Th(NO3)4 and 1% Ce(NO3)4 in distilled water was used as lighting fluid. A fabric sock dipped in this fluid was used to create the gas mantle. When lit, thorium caused the gas mantle to give a very bright white light [29]. Mischmetal, a complex alloy of rare earth metals, alloyed with iron is used as the flint (spark-producing agent) in cigarette lighters and similar devices.
Rare earths are now used for making magnets. In general, rare earths magnets are anisotropic, which means they have a preferred direction of magnetic orientation. Compared to isotropic magnets, anisotropic magnets retain better magnetization at zero external field, have better maximum energy product, i.e., the maximum product which can be obtained on the demagnetization curve, and have superior mechanical properties and corrosion resistance [30]. A powerful magnet made of neodymium, Nd2Fe14B, of magnetic energy 50MGOe was invented in 1984 [31]. Initially this Nd-Fe-B magnet was used in data processing and audio-visual equipment. Now Nd-magnets are used in medical appliances such as magnetic resonance imaging equipment, in electronics such as manufacture of computers, cell phones, etc.
Alloys of gadolinium have been used in magnetic refrigeration and have great prospects for cryogenic applications. Gadolinium-aluminum-garnet (GAG) has been used in magnetic refrigeration for cooling up to a temperature of 4K.
Garnets of some rare earths, such as samarium, gadolinium, and yttrium, are used in making bubble domain memories. The magnetic bubble is stable in the presence of a small field applied by a permanent rare earth magnet. This makes the bubble domain memory nonvolatile, that is the information in the memory is not lost when the power to the memory is off. Also bubble domain can be reduced to about one micrometer, which increases the storage density for information. Storage densities can be as high as one megabit per square centimeter.
Rare earth intermetallics, REM5 (where RE refers to a rare earth and M refers to one of the metals, Co, Ni, or Fe) have the capacity to absorb a large amount of hydrogen at room temperature. Such intermetallics are, therefore, used for hydrogen storage.
The rare earths, ceria and yttria are used as sintering aids in powder metallurgy. Mischmetal was used in the steel alloy for the Alaska crude oil pipeline. Cerium oxide or cerium rich rare earth oxide is used for glass polishing. Rare earths are also used in solid oxide fuel cells called ceramic fuel cells. Pure cerium has been used as an opacifier in ceramic glasses and CeS and yttria are used in making crucibles. Praseodymium is added to pigments to make them stable at high temperature. Cerium oxide is a key element in the catalytic convertors and is used to break down unburned hydrocarbons in automobiles.
Rare earths are also used in optics and phosphor industries. In laser glasses Nd acts as an activator. Glass fibers containing rare earths can transmit data over exceptionally large distances without booster stations. The phosphor in cathode ray tubes is Eu-doped yttrium vanadate, giving the red color. Other phosphors also contain rare earths. Cerium oxide is the catalyst used in self-cleaning ovens. Neodymium-yttrium-garnet and gadolinium-yttrium garnet are used in the manufacture of lasers as alternatives to ruby.
Rare earths have found applications in nuclear power industry. Gadolinium oxide has a high neutron absorption cross section and, therefore, is used in nuclear reactors to achieve a uniform neutron flux.
In this article only very few applications of rare earths are mentioned with a view to recognize the importance of rare earths. Many more applications are in existence and the potential for more applications is high. Intensive research for the use of rare earths in all fields is being pursued.
References
- Discovery of Promethium, ORNL Review 36, No. 1 (2003), http://www.ornl.gov/info/ornlreview/v36_1_03/article_02.shtml
- N. E.Holden, History of the Origin of the Chemical Elements and Their Discoverers, (Prepared for the 41st IUPAC General assembly in Brisbane, Australia June 29th - July 8th, 2001 BNL-NCS-68350-01/10-Rev; Last Updated: March 12, 2004), http://www.nndc.bnl.gov/content/elements.html
- D. Mendelejeff, On the Relationship of the Properties of the Elements to their Atomic Weights, 12, 405-406 (1869); translation by Carmen Giunta.
- E. Weil, Some Bibliographical Notes on the First Publication on the Roentgen Rays, Isis, Vol. 29, No. 2, 362-365 (Nov., 1938).
- H. G. J. Moseley, M. A., The High Frequency Spectra of The Elements, Phil. Mag., 1024 (1913), Phil. Mag., 703 (1914).
- Niels Bohr, The Structure of the Atom, Nobel Lecture, December 11, 1922,
- http://nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-lecture.pdf
- Ernest Rutherford, The Chemical Nature of the Alpha Particles from Radioactive Substances, Nobel Lecture, December 11, 1908, http://ca.geocities.com/chemmystryyy/rutherford-lecture.html
- G, Kirchhoff and R. Bunsen, Chemical Analysis by Observation of Spectra, Annalen der Physik und der Chemie (Poggendorff), Vol. 110, 161-189 (1860).
- J. Gadolin, Academic Radiology, 1996 Aug;3 Suppl 2:S165-9.
- P. van der Krogt, Cerium - Elementymology & Elements Multidict, http://www.vanderkrogt.net/elements/elem/ce.html
- Separation of Rare Earth Elements, American Chemical Society, National Historical Chemical Landmark (1999), URL at ACS.org.
- Nobel Lectures, Chemistry 1942-1962, The Nobel Prize in Chemistry 1951 Presentation Speech, http://nobelprize.org/nobel_prizes/chemistry/laureates/1951/press.html
- Wahid U. Malik, G. D. Tuli, R. D Madan, Selected Topics in Inorganic Chemistry, 296 (2006), S. Chand & Company Ltd., New Delhi.
- C. W. Thiel, Y. Sun, and R. L. Cone, Preprint version of article published in “Journal of Modern Optics” 49, 2399 (2002).
- C. K. Gupta and N. Krishnamurthy, Extractive Metallurgy of Rare Earths (2004), CRC Press.
- I. Maurits Kolthoff and P. J. Elving, Treatise on Analytical Chemistry - Vol. 13, 10 (1959)
- H. G. Byer, Inorganic Chemistry, 528 (1971), C. Scribner's sons.
- Y. Marcus and J. A. Marinsky, Ion Exchange and Solvent Extraction- 11 (1993).
- S. P. Sinha, Systematics and the Properties of the Lanthanides, 73 (1983), Springer.
- H. J. Issaq, A Century of Separation Science (2002), CRC Press.
- G. M. Ritcey and A. W. Ashbrook, Solvent Extraction: Principles and Applications to Process Metallurgy, Parts I and II, (1979), Elsevier.
- C. J. Heinink, Preparation of high purity rare earth metals, Research Chemicals Div., Nuclear Corp. of America Calf, http://stinet.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD0276748
- R. Lundin and J. R. Wilson, Rare Earth Metals Find Interesting New Uses, http://www.arris-intl.com/customer/Papers/remetalspaper.html
- T. Moeller, The Chemistry of the Lanthanides (1963), Reinhold Publishing Corp., New York.
- D. M. Yost, H. Russell, Jr., and C. S. Garner, The Rare Earth Elements and Their Compounds (1947), John Wiley & Sons, New York.
- F. H. Spedding. and A. H. Daane, eds., The Rare Earths, (1961), John Wiley & Sons, New York.
- J. Jensen and A. R. Mackintosh, Rare Earth Magnetism - Structures and Excitations, (1991) Clarendon Press, Oxford.
- J. B. Calvert, Rare Earths, http://mysite.du.edu/~jcalvert/phys/rare.htm
- Austrian History Notes, http://austriannotes.com/history/article.php?products_id=23
- I. E. Anderson, R. W. McCallum and M. J. Kramer, Support: DOE-BES and DOE-EE-FCVT Program, Contract: W-7405-ENG-82, Ames Laboratory (USDOE), Iowa State University, Ames, Iowa.
- A. Koji, K. Osamu, S. Tatsuya, I. Toshiyuki, and O. Ryuichi, Rare earth cast alloy permanent magnets and methods of preparation, United States Patent 5565043 (1984).
© 2015 Discover the World