Tobacco Radioactivity

Although by 1950 it was understood that smoke from burning tobacco contained radioactivity, its danger to tobacco smokers was then considered to be minimal. It was not until the mid-1960s that investigators accelerated their work in this area. Their new studies showed that radioactivity in tobacco smoke posed significant danger to tobacco smokers and to persons exposed to tobacco smoke from active smokers.
More Bad Stuff Found
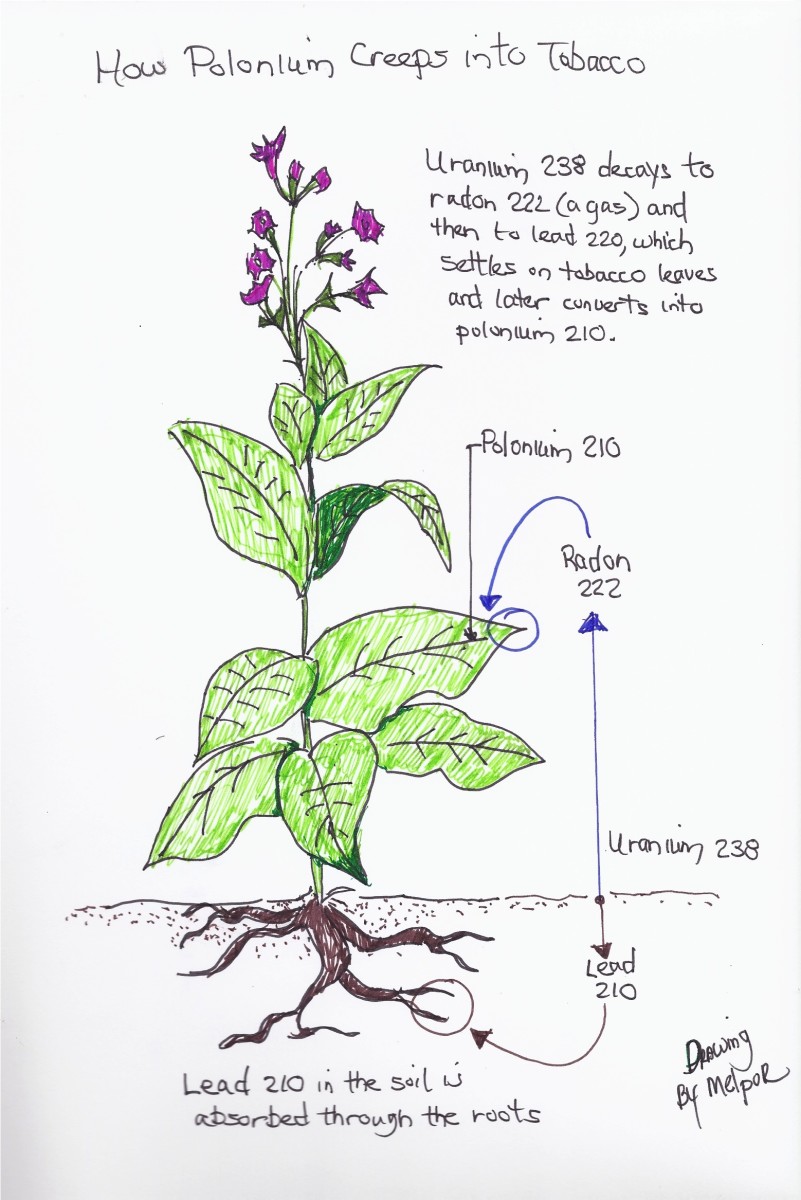
Observations now identified another two radioactive elements in tobacco smoke. These were lead-210 (210Pb) and polonium-210 (210Po), the latter being a descendant of the radioactive lead following its nuclear decay. Investigator Ed Martell explained that these two radioactive metals adsorb onto the leaves of growing tobacco from the air above heavily fertilized soils in which tobacco is grown. The source of this heavy-metal radioactivity, Martell stated, was largely apatite mineral from which agricultural fertilizers were made.
Apatite is rich in radioactive uranium and uranium's many post-decay radioactive elements.One of those decay products is radon-222 (and a lesser amount of radon-220), which in the gaseous state are present in the air under and around growing tobacco. There, radon can undergo radioactive decay, producing lead-210 and its daughter product, polonium-210. These two radioactive metals have been shown to "grab onto" dust particles and other aerosols in the air around the tobacco plants and, from there be taken up by sticky-surfaced trichomes (hairs) on the tobacco leaves. There, radioactivity remains throughout tobacco processing until it is released into the lungs and other organs of those who inhale tobacco smoke.
You Smoke it, You Own It
Those who inhale tobacco smoke also inhale smoke-borne tobacco “tars,” sticky substances that can adhere to the surface tissues in the breathing organs, such as the linings of bronchial tubes and tiny alveoli (air sacks) in the lungs. Tobacco's radioactive material, mixed in with the sticky tars, tends to lock onto breathing organ surfaces, particularly at locations where the bronchial tubes bifurcate (branch) and in the alveoli at tube ends, There the radioactive material sits, emitting radiation, until it eventually decays away to become non-radioactive lead metal.
A Little Goes a Long Way, and it Goes and Goes and Goes
The amount of radioactivity per tobacco cigarette, cigar,or pipe-load is very tiny, but, because of buildup of radioactive material within bronchi, bronchioles, and alveoli, radiation to exposed tissues increases over time. Lead-210 has a half-life of approximately 22 years – the time required for half of an available quantity of the radioactive lead to disintegrate, atom by atom, on its way to becoming the even more dangerously radioactive element, polonium-210. It is this radioactive polonium that actually causes the most damage from tobacco smoke inhalation.
How Does 5.3 Million Electron Volts Sound to You?
Radioactive polonium is continually produced by decay of its predecessor radioisotope, radioactive lead. This polonium-210 then disintegrates according to its own half-life schedule of essentially 138 days. Upon polonium's disintegration an alpha particle is emitted, and on rare occasion, a powerful gamma ray may be released as well. The alpha particle has 5.3 million electron volts of ionizing energy due to its relatively large mass and double-positive electrical charge.
Although alpha particles cannot travel very far in body tissue due to their mass (the same as a helium atom nucleus – 4 atomic mass units) and charge, they are very destructive to sensitive body tissues onto which lead-210 and its resulting polonium-210 are plastered. When and if the alpha particles reach and interact with cellular DNA or other cell "officiators," mutations can be produced, and those might lead to mutation defects toward cancer formation and other malformations.
Do You Really Need a Chest X-ray Every Day?
Thus it is that a person who smokes 20 or more tobacco cigarettes a day for 25 years receives an equivalent amount of ionizing irradiation as that person would get from one chest X-ray per day – every day – for the balance of the smoker's life. Tobacco does not emit X-rays, but the comparison here is to the X-ray equivalent radiation exposure from the body's burden of continuously emitting radioactive material it took on from years of tobacco smoking.
Keep on Reading, But You May Not Enjoy It
There is a great deal more to be discussed regarding the radioactivity of tobacco smoke and the dangers tobacco smoke poses to active and to passive tobacco smokers. This very short article only touches on some of the lesser-known aspects of tobacco smoke dangers. More should be reviewed and understood by everyone, smokers and non-smokers alike.
Reference: Cancer Risk in Relation to Radioactivity in Tobacco. Radiol Technol: 1996;67(3):217 (also see PubMed.gov)
The billboard image was photographed in Houston, Texas. Produced by some local high school students, it shows the cartoon character used by the state health department in its anti-smoking campaign, still on-going through the Internet Website, “DuckTexas.com.”
Eat Your Broccoli and Turnips
Someone asked me one time if it were “safe” to eat fruits and vegetables grown in soil which had been treated using the same types of chemical fertilizers used in tobacco growing. Think of that situation. Foods we swallow zip through our digestive tracts quickly and are gone. If they contained insoluble radioactive metals, such as polonium and lead, they would not hang around in the body long enough to do the degree of damage to tissues that can come from inhaling radioactivity into the breathing system; that is, unless the food is first dried and then smoked as people do with tobacco. Think “smoking 20 carrots a day for 25 years.” I believe I'd stay away from someone who did that, even if the carrots were not at all radioactive.
But you go ahead and have a nice day today. Eat your veggies as “Mama” taught you. That way your eyes will sparkle, your feet won't hurt, and you will always smell really good. Not like with smoking tobacco,OK?
Copyright 2014 GF Kilthau