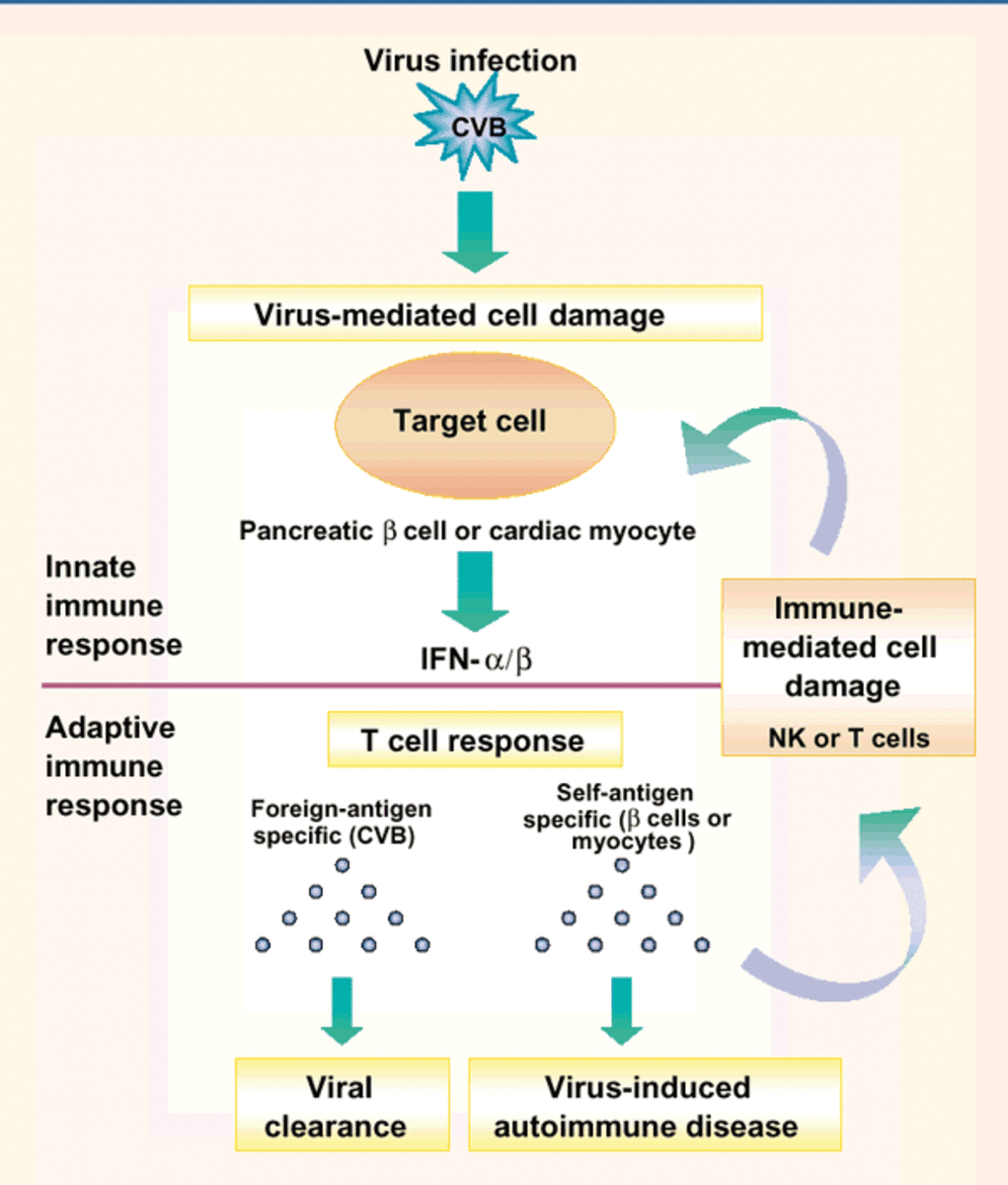
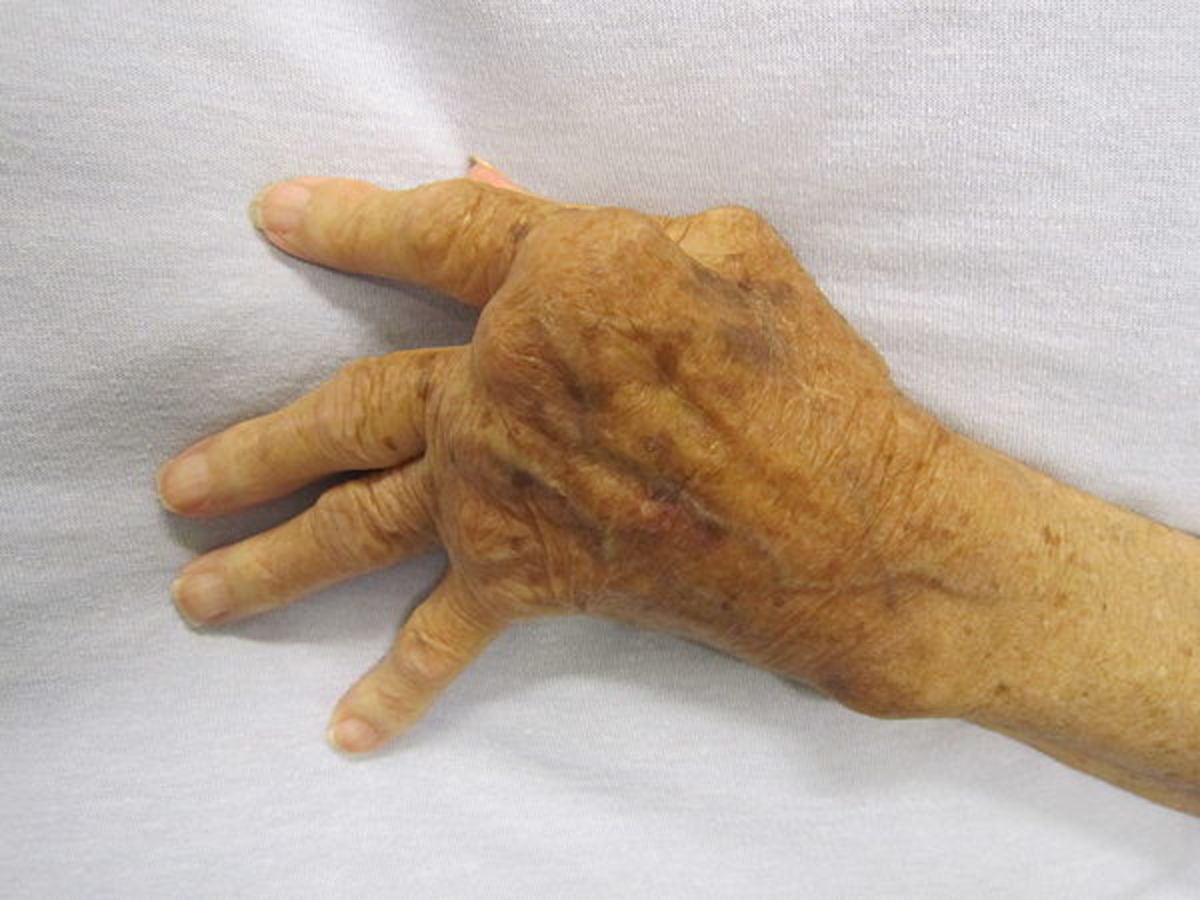
Biologic immunomodulators drugs for Autoimmune diseases; RA, Systemic Lupus & Crohn’s disease CD.
These biologic drugs are specifically engineered molecules designed to block particular immunologic activation steps involved in the pathogenesis of diseases such as rheumatoid arthritis, Lupus or psoriasis. These conditions involve the actions of various cellular components, including lymphocytes, macrophages and B cells, and secreted compounds, such as interleukins, tumor necrosis factor, and other cytokines. The agents themselves include monoclonal antibodies (MAbs) and fusion proteins, which are directed against proinflammatory cytokines, such as tumor necrosis factor (TNF), and the interleukin (IL)-1 receptor and selected cell surface markers on immune cells, such as cytotoxic T-lymphocyte-associated antigen 4 immunoglobulin (CTLA-4-Ig) and CD20.
Three monoclonal antibody (mAb) therapeutics had been granted their first approval in May 21 2014. Ramucirumab (Cyramza®), siltuximab (Sylvant®) and vedolizumab (EntyvioTM) were approved by the Food and Drug Administration (FDA) on April 21, April 23 and May 20, 2014, respectively. Ramucirumab, which targets human vascular endothelial growth factor receptor- 2, is indicated for the treatment of advanced gastric cancer or gastro-esophageal junction adenocarcinoma, as a single-agent after prior fluoropyrimidine-or platinum-containing therapy. The dosage is 8 mg/kg administered intravenously (iv) every 2 wk for these indications.
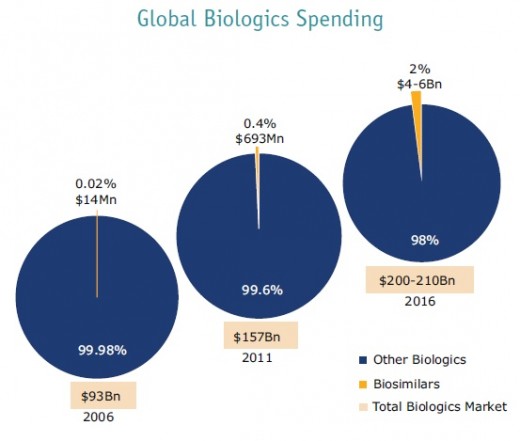
Biologics are classified as specialty drugs, which are the fastest-growing segment of drug expenditures in the U.S. In 2011, specialty medications accounted for 17.6% of total pharmacy costs. The actual growth is masked because about 47% of specialty drugs are billed under medical benefits. Moreover, the emerge of new autoimmune diseases are driving up the costs of these specialty administered drugs. although new oral biologic therapies may offer simplified administration (tofacitinib), they are still more costly than more commonly prescribed therapies (around $800 per 100mg).
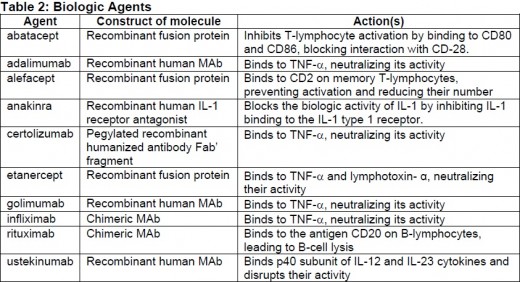
Biologic drugs are genetically engineered protein molecules that block the proinflammatory cytokines TNF-a (etanercept, infliximab, adalimumab, certolimumab pegol, golimumab), Interleukin (IL)-1 (anakinra), IL-6 (tocilizumab), and IL-12 and IL-23 (ustekinumab), deplete peripheral B cells (rituximab), bind to CD80/86 on T-cells to prevent the costimulation needed to fully activate T-cells (abatacept), or inhibit janus kinase (tofacitinib). These drugs may be effective when other non- biologic disease-modifying antirheumatic drugs (DMARDs) fail to achieve adequate response, but are considerably more expensive to use.
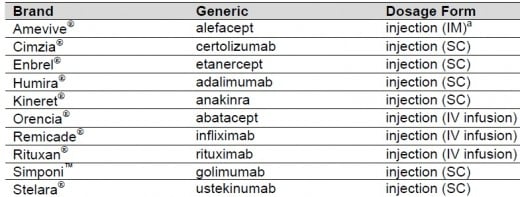
Biologic agents are classified as anti-tumor necrosis factor (TNF) or non-TNF biologics. Anti-TNF biologics include adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), golimumab (Simponi) and infliximab (Remicade). Non-TNF biologics include abatacept (Orencia), anakinra (Kineret), rituximab (Rituxan), tolcilizumab (Actemra), tofacitinib (Xeljanz), ustekinumab (Stelara) and Alefacept (Amevive).
Biologic immunomodulators drugs are used for the treatment of serious inflammatory disorders and autoimmune diseases such as rheumatoid arthritis (RA), psoriasis (PS), psoriatic arthritis (PA), ankylosing spondylitis (AS), juvenile idiopathic arthritis (JIA), Crohn’s disease (CD), and ulcerative colitis (UC).
On March 20, 2014, the EMA’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, which recommended the granting of a marketing authorization for siltuximab.
siltuximab, SYLVANT (Anti-interleukin (IL)-6) is prescribed in the US for treatment of patients with multicentric Castleman disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative. Dosing is 11 mg/kg given over 1h by iv infusion every 3 wk. 100mg of siltuximab costs around $918.
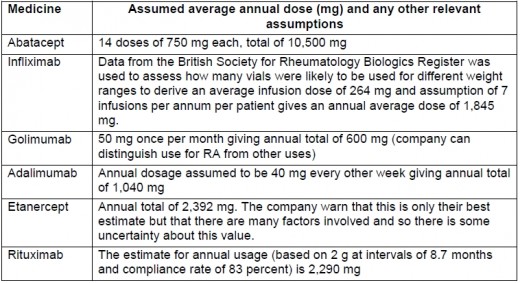
The most commonly used non-biologic DMARDS include hydroxychloroquine (HCQ), leflunomide (LEF), methotrexate (MTX), minocycline, or sulfasalazine. Gold, cyclosporine, and azathioprine are infrequently used.2 Biologic agents have no toxicity that requires laboratory monitoring, but have increased risk for infection.
Methotrexate, mitoxantrone, azathioprine, cyclophosphamide, mycophenolate mofetil and azathioprine are immunosuppressive drugs.
Methotrexate is labeled to be used in combination with Remicade, Rituxan, and Simponi for treatment of rheumatoid arthritis (RA) and SLE.
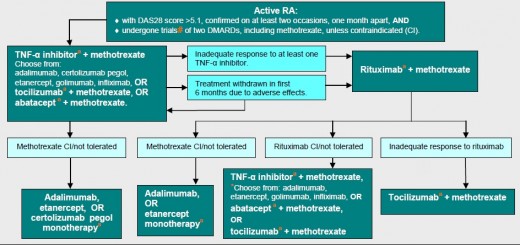
Adalimumab, certolizumab pegol or etanercept are used as monotherapy if the patient has a contraindication (CI) to methotrexate.
certolizumab pegol (Cimzia, UCB Pharma Limited) is prescribed for treating Tumour necrosis factor (TNF)-alpha inhibitors are used for the treatment of rheumatoid arthritis (RA) in terms of reducing the risk of joint damage.
The Disease Control and Prevention (CDC) estimates that in the united states alone, more than 23,00 people die as a result of antibiotic-resistant infections each year. the cost of antimicrobial resistance has been estimated to $20 billion. as a result, the CDC now classifies antimicrobial resistance as one of the most serious health threats worldwide.
Vedolizumab is approved to treat adult patients with moderate to severe ulcerative colitis (UC) and adult patients with moderate to severe Crohn disease (CD) when one or more standard therapies (corticosteroids, immunomodulators, or tumor necrosis factor blocker medications) have not resulted in an adequate response. The humanized IgG1 mAb, which targets α4β7 integrin, is Fc-engineered to silence effector functions.
Vedolizumab (Entyvio) is also recommended to the UK NHS as a possible treatment for adults with moderate to severe ulcerative colitis (The National Institute for Health and Care Excellence code TA342) date released in the UK 24/06/2015.
The UK's National Institute for Health and Care Excellence Biologic Drug Technology Appraisals
TA130 Adalimumab, etanercept and infliximab for treatment of RA
TA186 Certolizumab pegol for treatment of RA
TA195 Adalimumab, etanercept, infliximab, rituximab and abatacept for treatment of RA after failure of a TNF-α inhibitor.
TA225 Golimumab for treatment of RA after failure of previous DMARDs.
TA247 Tocilizumab for treatment of RA.
TA280 Abatacept for treatment of RA after failure of conventional DMARDs
_________
Vedolizumab is given to patients that have failed either "conventional therapy" (which includes steroids and immunosuppressants) or TNFá-antagonists.
The most commonly used Disease-modifying anti-rheumatic drugs (DMARDs); azathioprine
(Imuran®), cyclosporine (Neoral®), gold therapy, sodium aurothiomalate (Myochrysine®),
hydroxychloroquine (Plaquenil®), leflunomide (Arava®), penicillamine (Cuprimine®) sulfasalazine (Salazopyrin®, generics), tacrolimus (Prograf®) and many others.
Certolizumab pegol in combination with methotrexate (MTX) is prescribed for the treatment of moderate to severe active RA in adult patients when the response to DMARDs including MTX has been inadequate. It is also used as monotherapy in the case of intolerance, side effects or contraindications to MTX. The drug is designed to reduce the rate of progression of structural joint damage as measured by x-ray. it is not meant to cure any patient with autoimmune disease.
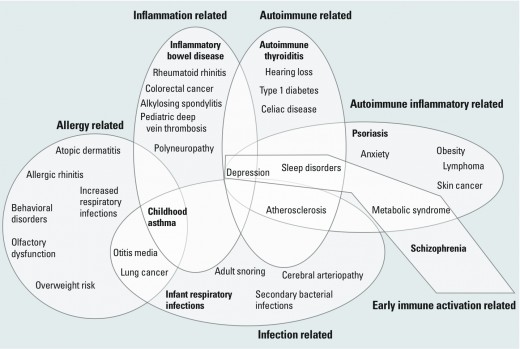
Reports of biologic drugs side effects;
Autoimmune Hepatitis triggered by Adalimumab and allergic reactions after various Anti-TNFα therapy agents in a patient with Rheumatoid Arthritis.
Liver enzyme abnormalities are commonly observed in patients with rheumatoid arthritis (RA), inflammatory bowel diseases (IBD), spondyloarthropathy and psoriasis, who are generally treated with anti-TNFα drugs.
many cases of TB had been reported to the FDA’s Adverse Event Reporting System in association with infliximab and 38 cases of patients (with RA) who have developed TB worldwide while being treated with etanercept.
Tuberculosis (TB) is the most commonly reported opportunistic infection associated with the TNF-α blocking drugs.
In most cases the TB infections arise from the reactivation of latent infection and usually occur within the first 2-5 months of treatment.
MTX hepatotoxicity is a common complication of long-term treatment for rheumatoid arthritis patients. It is associated with mild liver enzyme elevation and related to the long-term use of Methotrexate therapy.
Increases in aminotransferases (transaminitis) are potential major adverse reactions seen with long- term use of methotrexate (MTX).
Methotrexate and Cyclophosphamide are also used for the treatment of Autoimmune inner ear disease (AIED) and Lupus.
Methotrexate is a Hepatotoxic drug, this means the patient is at risk from liver injury on long term use of MTX. Methotrexate (MTX) has been available for clinical use since 1951.
Studies have showen that Methotrexate elevated liver enzymes 2-3 times greater than the normal range.
Methotrexate, mitoxantrone, azathioprine, cyclophosphamide, mycophenolate mofetil and azathioprine are immunosuppressive drugs.
Active TB and reactivation of latent TB infection (LTBI) have been reported after use of biologic drug therapy in pateints with autoimmune diseases; RA, SLE, CD.
long term use of vedolizumab drugs could potentially cause progressive multifocal
leukoenceophalopathy (PML) a rare neurological disorder.
Procainamide and Hydralazine can induce autoantibodies and lupus-like disorders in patients. Penicillamine has been associated with myasthenia gravis and a-methyldopa is known to cause a form of haemolytic anaemia.
Treatment of rheumatoid arthritis with abatacept (Orencia), tocilizumab (Actemra) and Infliximab was reported as the main cause in the majority of cases that developed liver injury.
It is important to recognise that certolizumab pegol and methotrexate are effective in reducing signs and symptoms in patients with rheumatoid arthritis and lupus but these biologic drugs do not address the underlying root cause of autoimmune diseases.