Interesting facts and history of abortion pill
The invention and discovery of abortion pill
The invention: RU-486 was the first commercially available drug that prevented fertilized eggs from implanting themselves in the walls of women’s uteruses.The people behind the invention:
Étienne-Émile Baulieu , a French biochemist and endocrinologist
Georges Teutsch, a French chemist
Alain Bélanger, a French chemist
Daniel Philibert, a French physicist and pharmacologist
Developing and Testing
In 1980, Alain Bélanger, a research chemist, was working with Georges Teutsch at Roussel Uclaf, a French pharmaceutical company. Teutsch and Bélanger were interested in understanding how changes in steroids affect the chemicals ability to bind to their steroid receptors. (Receptors are molecules on cells that can bind with certain chemical substances such as hormones. Receptors therefore act as connecting links to promote or prevent specific bodily activities or processes.) Bélanger synthesized several steroids that bonded to steroid receptors. Among these steroids was a compound that came to be called "RU-486." Another member of the research project, Daniel Philibert, found that RU-486 blocked the activities of progesterone by binding tightly to the progesterone receptor. Progesterone is a naturally occurring steroid hormone that prepares the wall of the uterus to accept a fertilized egg. Once this is done, the egg can become implanted and can begin to develop. The hormone also prevents the muscles of the uterus from contracting, which might cause the uterus to reject the egg. Therefore RU-486, by acting as a kind of shield between hormone and receptor, essentially stopped the progesterone from doing its job. At the time, Teutsch's group did not consider that RU-486 might be useful for deliberately interrupting human pregnancy. It was Étienne-Émile Baulieu, a biochemist and endocrinologist and a consultant for Roussel Uclaf, who made this connection. He persuaded the company to test RU-486 for its effects on fertility control. Many tests were performed on rabbits, rats, and monkeys; they showed that, even in the presence of progesterone, RU-486 could prevent secretory tissue from forming in the uterus, could change the timing of the menstrual cycle, and could terminate a pregnancy- that is, cause an abortion. The compound also seemed to be nontoxic, even in high doses.
In October of 1981, Baulieu began testing the drug with human volunteers. By 1985, major tests of RU-486 were being done in France, Great Britain, The Netherlands, Sweden, and China. When a relatively low dose of RU-486 was given orally, there was an 85 percent success rate in ending pregnancy; the woman's body expelled the embryo and all the endometrial surface. Researchers found that if a low dose of a prostaglandin (a hormonelike substance that causes the smooth muscles of the uterus to contract, thereby expelling the embryo) was given two days later, the success rate rose to 96 percent. There were few side effects, and the low doses of RU-486 did not interfere with the actions of other steroid hormones that are necessary to keep the body working. In the March, 1990, issue of The New England Journal of Medicine, Baulieu and his coworkers reported that with one dose of RU-486, followed in thirty-six to forty-eight hours with a low dose of prostaglandin, 96 percent of the 2,040 women they studied had a complete abortion with few side effects. The women were monitored after receiving the prostaglandin to watch for side effects, which included nausea, vomiting, abdominal pain, and diarrhea. When they returned for a later checkup, fewer than 2 percent of the women complained of side effects. The researchers used two different prostaglandins; they found that one caused a quicker abortion but also brought about more pain and a longer period of bleeding.
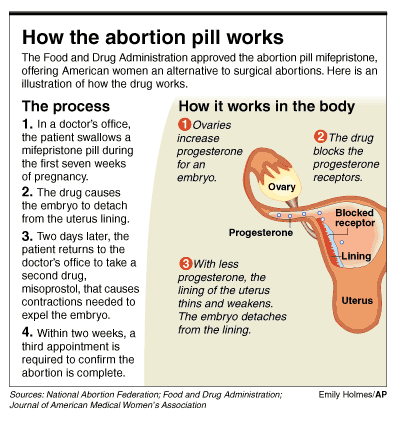
Using the drug
In September, 1988, the French government approved the distribution of RU-486 for use in government-controlled clinics. The next month, however, Roussel Uclaf stopped selling the drug because people opposed to abortion did not want RU-486 to be available and were threatening to boycott the company. Then, however, there were threats and pressure from the other side. For example, members of the World Congress of Obstetrics and Gynecology announced that they might boycott Roussel Uclaf if it did not make RU-486 available. The French government, which controlled a 36 percent interest in Roussel Uclaf, ordered the company to start distributing the drug once more. By the fall of 1989, more than one-fourth of all early abortions in France were being done with RU-486 and a prostaglandin. The French government began helping to pay the cost of using RU-486 in 1990.Testing for approval of RU-486 was completed in Great Britain and The Netherlands, but Roussel Uclaf's parent company, Hoechst AG, did not try to market the drug there or in any other country outside France. (In the United States, government regulations did not allow RU-486 to be tested using government funds.) Medical researchers believe that RU-486 may be useful not only for abortions but also in other ways. For example, it may help in treating certain breast cancers and other tumors. RU-486 is also being investigated as a possible treatment for glaucoma-to lower pressure in the eye that may be caused by a high level of steroid hormone. It may be useful in promoting the healing of skin wounds and softening the cervix at birth, easing delivery. Researchers hope as well that some form of RU-486 may prove useful as a contraceptive- that is, not to prevent a fertilized egg from implanting itself in the mother's uterus but to prevent ovulation in the first place.

Impact
Groups opposed to abortion rights have spoken out against RU- 486, while those who favor the right to abortion have urged its acceptance. The drug has been approved for use in China as well as in France. In the United States, however, the government has avoided giving its approval to the drug. Officials of theWorld Health Organization (WHO) have argued that RU-486 could prevent the deaths of women who undergo botched abortions. Under international law,WHOhas the right to take control of the drug and make it available in poor countries at low cost. Because of the controversy surrounding the drug, however,WHOcalled for more testing to ensure that RU-486 is quite safe for women.