Diet Drugs: Controversy and Complications

The purpose of this article is to prove or disprove my theory that the majority of weight loss drugs have had side effects bad enough to have them pulled off the market by the FDA. Let's see how my theory plans out.
There are two major types of weight loss pills out there- pharmaceuticals and dietary supplements. The former require FDA approval and thus proof of their efficacy and safety while dietary supplements don’t require the same proof as drugs, however the FDA can recall them if the product receives adverse event reports from consumers using the supplement. For attention-span purposes I will only go into diet drugs here.
As much as I am an advocate for the old fashion weight loss methods- eating healthy and exercising- I understand there are reasons why weight loss pills and supplements are necessary. Ultimately these people deserve safe weight loss drugs but based on my research there are not many options that don't come with the potential of serious health complications.
Diet drugs are only recommended for obese people (not those looking to only lose a few pounds) and are for use in addition to, not instead of, a well-balanced diet and exercise. Also several are recommended by the FDA only if serious cardiovascular risks are already present and the person needs substantial weight loss to reduce these risks. Lastly the FDA recommends these drugs be resorted to only when diet and exercise alone have not been successful. The health consequence of obesity is the risk it imparts on an individual’s cardiovascular health. The FDA sees weight loss drugs as a potential way to help an individual lose weight and thus removing the strain on their cardiovascular system. Ultimately this means weight loss drugs could aid in the prevention of death from cardiovascular disease.
It turns out diet drugs do have a notorious history with the FDA. It seems each time one is presented it gets mixed reviews from the approval committee on whether it is safe enough or efficacious enough to put on the market. In previous articles of mine about pharmaceuticals a major theme is the benefit to risk ratio of an individual drug. Diet pills are somewhere in the middle in terms of being a life-saving drug which is directly related to its risk to benefit ratio. Ultimately the FDA’s reason for approving these drugs is for the benefits that the lost weight will have on the person’s cardiovascular system. But interesting enough it is almost always cardiovascular side effects that result in these drugs being banned.
The guidelines in place are a diet pill should allow the intervention group to lose 5% more weight than the placebo group in a controlled trial that lasts one year (or at least a greater portion of patients in the trials need to loss 5% of their weight at one year than those that do not). Obesity is defined by Body Mass Index where a BMI of 30 or above is considered obese, for example a person who is 5 feet 7 inches and weighs 191 pounds has a BMI of 30 and is technically obese. Put this example person in a diet pill trial and they would only need to lose 5% of their total body weight, 9.5 pounds, in order for the drug to be deemed acceptable as far as the FDA is concerned. I personally don’t think 9.5 pounds in one year off of a 200 pound person, who according to the recommendation should be eating healthy and exercising as well, is really worth the risk these drugs can impart on the user.
Diet Drug Timeline

An interesting layer to the FDA-diet drugs story is the timeline, discussed in detail in an article by Eric Coleman in the Annals of Internal History (2005). 1938 marked the year when the FDA required drugs on the US market be proven to be safe as part of the Federal Food, Drug, and Cosmetic Act. This act was amended in 1962 to include the proof of the drug’s efficacy (It seems obvious that the US public deserves drugs that are both safe and do what they are advertised as doing. It is crazy to think the efficacy part was added on as late as the 60s). Certain diet drugs slid onto the scene with minimal scrutiny while the FDA was enacting these rules and establishing their guidelines for the benefit to risk ratio of weight loss drugs. Granted the FDA did go back and look at research conducted on all drugs approved before the 1962 amendment to see how efficacious they were. Amphetamine, the first major player in diet drugs, was ultimately shown to only have ‘possible effectiveness’ for weight loss and was questionable in safety due to its potential addictiveness and reported psychosis it caused. However all of this negative press came too late-amphetamine widespread usage was already firmly cemented in American culture for a variety of indications including general mental health purposes. Meanwhile it spawned several amphetamine-like compounds including Phentermine.
Phentermine is known as a sympathomimetic drug, this class of drugs mimic the sympathetic nervous system. Ultimately it stimulates the hypothalamus to decrease one’s appetite by conveying a feeling of being full. It is an appetite suppressant approved for short-term use of weight loss.
These similar compounds, amphetamine congeners, were supposed to lack amphetamine’s less desirable traits. Phentermine was approved in 1959, and the aforementioned FDA review showed amphetamine congeners to be ‘effective but’ meaning there was either a more efficacious or safer alternative out there. This lead to the FDA limiting the recommended use of all of these drugs in anti-obesity treatment to short-term use only and made the manufacturers clearly label the drugs as being potential addictive.
Cardiovascular System Physiology
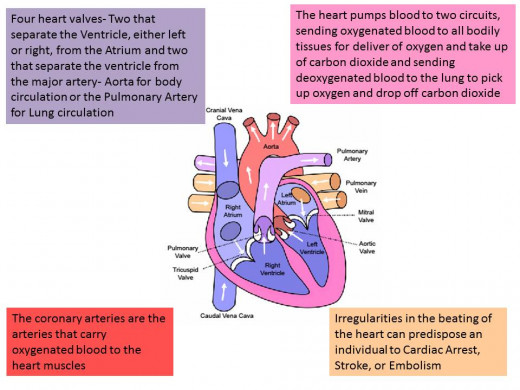
Although met with a decline in use when these amphetamine-like drugs were restricted and reprimanded by the FDA in the early 70s, this isn’t the end of the Phentermine story. Most people are familiar with the Fen-Phen craze in the early 1990s, prompted by studies using a combination of Fenfluramine and Phentermine claiming an impressive weight loss seen by research subjects. Both drugs were approved by the FDA for individual use but the combination of them had not been tested for safety and efficacy and thus this was known as off-label use of these drugs. Fenfluramine was approved by the FDA in 1973; it works by increasing levels of serotonin which in turn signal the hypothalamus to convey a feeling of being full. Dexfenfluramine, as the name suggests it is an isomer of fenfluramine, was approved in 1996 and also incited somewhat of a craze when put together, again in an off-label manner, with Phentermine. Both Fen-Phen and Dexfen-phen were deemed unsafe by the FDA after many reports of valvular heart disease. Fenfluramine and Dexfenfluramine were found to be the culprits in these two separate drug combinations, both were pulled from the market in 1997 while Phentermine remained for use on its own.
Cardiovascular Complications seen in Diet Drugs
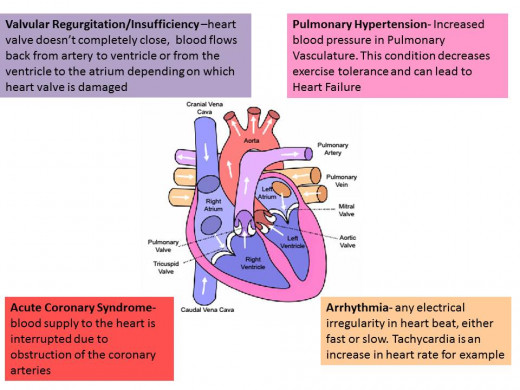
In 2012 Phentermine once again re-invented itself on the weight loss drug scene, this time as a component of Qsymia an FDA-approved combination drug along with the antiepileptic drug Topiramate. This drug as many of the others in this tale didn’t receive a standing ovation from the FDA. Its approval ultimately hinged on the promise of limited distribution by the manufacturer (Vivus Inc.) and future studies looking into the cardiovascular effects of the drug. Another drug was approved in 2012, Belviq which activates a specific serotonin receptor allowing for satiety. Another piece to this story is Sibutramine- a serotonin and norepinephrine reuptake inhibitor. It first appeared on the market in 1997 by the name of Meridia. Shortly after its approval the FDA and corresponding drug agencies in other parts of the world began receiving adverse drug event reports which included several cardiovascular-related deaths. At first the FDA saddled the drug with its sternest warning, the black box warning on the drug's label, but ultimately just pulled it all together in 2010.
Neurotransmitter role in Satiety and the Cardiovascular System

Diet Drug's Mechanism of Action
Trade name
| Drug name
| Mechanism of Action
|
|---|---|---|
Amphetamine
| Acts on norephinephrine and at higher doses dopamine as well
| |
Adipex -P, Obenix, etc.
| Phentermine
| Acts on norephinephrine (dopamine and serotonin to a lesser extent)
|
Pondimin
| Fenfluramine
| Increases Serotonin levels, sympathomimetic
|
Redux
| Dexfenfluramine
| Serotonin reuptake Inhibitor
|
Meridia
| Sibutramine
| Dual Norepinephrine and Serotonin Reuptake Inhibitor
|
Alli- over the counter; Xenical-prescription only
| Orlistat
| Gastrointestinal Lipase Inhibitor
|
Qsymia
| Fenfluramine + Topiramate
| Acts to increase Serotonin through fenfluramine
|
Belviq
| Lorcaserin
| Serotonin 2C Receptor Agonist
|
Orlistat, approved in 1999, works by inhibiting enzymes in the gastrointestinal system that break down fat into smaller components. The smaller components can then be absorbed into the body, whereas if triglycerides-the fats in question-remain intact they will be sent packing as waste.
When will we learn?
It seems like every drug with a similar mode of action allowing for weight loss comes with dangerous side effects messing with the cardiovascular system. These complications aren't even a big surprise seeing as these neurotransmitter targets have effects on both satiety, the intended mechanism, and the cardiovascular system. But every time a new drug comes out it gets approved by the FDA, then begins to cause problems in the general public and is pulled from the market. Shouldn’t there be some kind of guilty by association clause in FDA approval where a similar acting diet drug would need to jump through additional hoops before it is put on the market? In my research the only drug of a different overall mechanism from the ones detailed here was Orlistat. I'd like to think this indicates the tides are turning and the pharmaceutical industry is moving away from these SNS and CNS targetting drugs which can impart dangerous CV risk as well. But the last two drugs to the table target serotonin, as did fenfluramine and dexfluramine. Hopefully history won't repeat itself this time around in respect to these new drugs and hopefully current research is focused on areas other than multifunctioning neurotransmitters.
Resources
Colman E. History of medicine. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Annals Of Internal Medicine [serial online]. September 6, 2005;143(5):380-385.
Ioannides-Demos L, Proietto J, McNeil J. Pharmacotherapy for obesity. Drugs [serial online]. May 15, 2005;65(10):1391-1418.
Other resources available on request



