FENTANYL PRESCRIPTION LIST AND INFORMATION
Fentanyl Discovery and Development
Fentanyl is an extremely potent narcotic analgesic (pain reliever) first discovered and synthesized by Dr. Paul Janssen in 1960. Initially introduced as a general anesthesic, fentanyl has since been developed into a variety of prescription medication formulations utilized for the treatment of severe and chronic painful conditions including breakthrough cancer pain. Fentanyl is considered a schedule II narcotic in the U.S., meaning that it has a very high potential for addiction or abuse. Fentanyl is also very dangerous if used improperly. For these reasons, I am going to write a brief article describing the proper use and potential side-effects of all of the currently available fentanyl-containing prescription products typically available for outpatient use.
The advice and information about fentanyl products contained in this article is intended to help patients and prescribers, but is not intended to replace your doctor's advice or the manufacturers dosing guidelines. In this article I will review the current prescribing information and common side-effects associated with the 6 currently available fentanyl prescription drugs for outpatient (home) use.
The 6 prescription fentanyl products reviewed below include:
- Abstral
- Actiq
- Duragesic
- Fentora
- Lazanda
- Onsolis
On Micrograms, a side note: Fentanyl doses are measured in "micrograms" (mcg). That's small! Most medicines are measured in milligrams (1 milligram = 1000mcg). Just for reference, a grain of sand is about 15mcg. So an "average" dose of fentanyl of, say 400mcg, has the equivalent of about 27 grains of sand of fentanyl. By way of comparison, an 800mg dose of ibuprofen contains about 53,333 grains of sand of ibuprofen. The point? Fentanyl is very potent!

1. ABSTRAL - fentanyl sublingual tablets
Abstral is a fentanyl tablet intended for the treatment of breakthrough cancer pain (BTcP). Abstral is a "sublingual" tablet, meaning it is intended to be placed under the tongue and dissolved. Abstral is rapidly absorbed and acheives maximum blood levels within 30-60 minutes. Abstral, manufactured by ProStrakan (a UK pharmaceutical company), received U.S. marketing approval in January 2011.
Abstral, like all fentanyl products, is intended ONLY for patients that are already accustomed to narcotic use (AKA "opioid tolerant" patients). This is because the extreme potency of fentanyl can cause fatal suppression of the central nervous system in patients not already tolerant to opioids.
Abstral is currently available in 6 strengths:
- 100mcg
- 200mcg
- 300mcg
- 400mcg
- 600mcg
- 800mcg
Several important things to remember about taking Abstral:
- Abstral is for breakthrough cancer pain only. You must be taking a regular "around the clock" narcotic for pain (like morphine or oxycodone) while taking Abstral.
- Because of the risks associated with rapidly released fentanyl, patients prescribed Abstral will need to be enrolled in a special program to ensure you understand the risks and how to use it safely (known as the REMS program).
- Abstral is intended only for adults 18 years and older.
- A single-tablet Abstral dose is taken for breakthrough pain. Another dose is typically allowed in 30 minutes if necessary. After 2 doses, another dose may not be used for 2 hours. Always keep Abstral in the blister-pack containers it comes in, until you need it.
- Allow a tablet to completely dissolve under the tongue before drinking any water.
Possible Side Effects Include: Significant drowsiness, dizziness. Do not drive. Also, nausea, constipation, headache. If you experience difficulty breathing - contact your healthcare provider (or call 911) immediately.

2. ACTIQ - fentanyl lollipops
Fentanyl lollipops? That may sound rather bizarre, and they look nothing like candy lollipops, but the availability of fentanyl in this formulation was a great step forward in managing breakthrough cancer pain. Actiq was approved by the FDA in 1998 and is marketed by Cephalon pharmaceuticals. Actiq is available generically also.
Concerns have been raised by the apparently large proportion of prescriptions written for Actiq that are not for breakthrough cancer pain, considered "off label" prescribing.
Actiq (sometimes referred to as Actiq "lozenges") is available in 6 "berry flavored" strengths:
- 200mcg
- 400mcg
- 600mcg
- 800mcg
- 1200mcg
- 1600mcg
About Using Actiq:
- As with all fentanyl products, use ONLY if you are opioid tolerant (i.e. are currently taking other opioids for pain)
- Actiq is only intended for adults age 16 and older
- Patients prescribed Actiq will need to be enrolled in the safety "REMS" program to be sure they understand the risks of fentanyl
- Actiq comes on a white stick and the end is placed between the lower cheek and gum and slowly sucked to release the medication. For best results, allow the medication to be released over 15 minutes.
- Typically, if pain relief is not achieved 15 minutes AFTER finishing the Actiq dose, another dose may be adminisered. However, after the second dose, you must wait 4 hours to use the medication again.
Common side-effects include: Nausea, vomiting, dizziness, drowsiness, headache, constipation. Note: Breathing difficulty should be reported to a healthcare provider right away.

FDA Warning
The FDA recently issued a public warning about the importance of careful disposal of used Duragesic patches. There have been at least 10 cases in which young children have accidentally applied a "used" patch to themselves resulting in a fatal overdose. The recommended disposal method is to fold the sticky sides of the patch together and flush it down the toilet.
3. DURAGESIC - fentanyl transdermal patch
Originally approved by the FDA in January of 2005, Duragesic patches became the first form of fentanyl which patients could administer to themselves (i.e. a non-injectable form). Duragesic patches are different than every other prescription fentanyl preparation in three ways:
- Duragesic patches deliver fentanyl through a topical patch applied to the skin, changed every 72 hours.
- Duragesic patches are designed for slow-release maintenance pain control, not for immediate breakthrough pain.
- Duragesic is approved for more "general" pain relief, rather than simply being restricted to cancer-related pain. The official language used to describe its approved indication is "for management of persistent, moderate to severe, chronic pain" the requires (a) continuous around-the-clock opioid administration and (b) for pain that cannot be managed by other means.
Like other fentanyl preparations, Duragesic should only be used by patients already tolerant to opioids (i.e. patients who have been taking narcotic pain relievers already). Basically, if your body is not used to narcotics already, Duragesic is TOO strong for you. For practical purposes, we consider a patient "tolerant" if they have been taking at least 60mg of morphine daily, 30mg of oxycodone daily or 8mg of hydromorphone daily.
Duragesic patches are available in 5 strengths:
- Duragesic 12mcg (actually delivers 12.5mcg/hour)
- Duragesic 25mcg
- Duragesic 50mcg
- Duragesic 75mcg
- Duragesic 100mcg
Using Duragesic Patches:
Duragesic patches should be applied to a clean, hairless (ideally), area of skin on the upper body such as the chest, back, flank or outer arm. The skin should be clean, dry and non-irritated or broken.
Duragesic patches should be removed from the pouch immediately before application to the skin. Be careful not to accidentally cut or tear the patch (such patches cannot be used). Press firmly onto the skin for at least 30 seconds. If necessary, first aid tape can be used to hold the patch in place.
Duragesic patches should remain in place for 72 hours. In some cases, patients will need to change the patch every 48 hours. After 72 hours the patch should be removed, folded in half (adhesive sides together), and disposed of in a toilet. A new patch should then be applied to a different location (to prevent skin irritation). It is okay for the patch to get wet (shower) but patients should avoid exposing the patch on the skin to excessive heat (e.g. heating pads).
Side Effects: Nausea, vomiting, constipation, drowsiness, dry mouth, sweating. Note: Difficulty breathing should be immediately reported to a health care practitioner.

4. FENTORA - fentanyl buccal tablet
Fentora is a fentanyl tablet which is intended to dissolve in the patient's mouth when placed between the upper gums and cheeck. Fentora is indicated for the management of breakthrough cancer pain in patients who are already opioid tolerant (i.e. already take other narcotics to manage chronic cancer pain). Fentora was approved for use in the U.S. by the FDA in September 2006 and is marketed by Cephalon pharmaceuticals (they also market Actiq).
Fentora tablets come in 6 different strengths:
- 100mcg
- 200mcg
- 300mcg
- 400mcg
- 600mcg
- 800mcg
How to use Fentora:
- A Fentora tablet is removed from the blister pack and immediately placed between the gum and cheek beside your upper molar. Allow the tablet to slowly dissolve (without sucking or chewing). This usually takes 15-25 minutes.
- If pain relief is not sufficient in 30 minutes a second dose may be administered. After this second dose, a patient may not use Fentora again for 4 hours.
- Your Fentora dose may need to be slowly increased under the direction of your doctor to properly manage your pain.
- Fentora contains no sugar and is unflavored.
Side Effects:
Nausea, vomiting, dizziness, confusion. Call your doctor if you experience difficulty breathing or experience excessive drowsiness as this may mean the dose it too high for you.

5. LAZANDA - fentanyl nasal spray
The latest addition to the fentanyl family of prescription drugs is a nasal spray called Lazanda. Approved by the FDA in June 2011, Lanzanda is a product of Archimedes Pharma. Lazanda is indicated for the relief of breakthrough cancer pain in adult patients age 18 and older. As with other forms of fentanyl, Lazanda may only be used by patients already tolerant to opioids. Prescribers, patients and pharmacies must be enrolled in the manufacturers REMS program to ensure they are trained and qualified to properly instruct patients to safely use Lazanda. The pharmacy will enroll patients who present a prescription for the first time.
Lazanda is the only fentanyl nasal spray available, and currently comes in 2 strengths:
- 100mcg/dose
- 400mcg/dose
Each glass bottle of Lazanda contains just 8 doses (after priming). A box of Lazanda contains either 1 bottle or 4 bottles. All patients should begin with the 100mcg dose/spray.
How To Use Lazanda:
- Lazanda nasal spray must be "primed" before the first use. You will do this by spraying Lazanda into the supplied pouch until a "green" solid line appears in the counter window.
- Administer Lazanda by inserting the tip into 1 nostril, holding the other nostril closed, and give 1 spray of the bottle. NEVER use more than 1 spray unless instructed to do so by your doctor. After your dose, you must wait 2 hours to administer another dose.
- Lazanda must be discarded if (1) it has been 5 days since you last used it or (2) it has been 14 days since initial priming.
Side Effects:
Potential side effects with Lazanda are similar to all of the other rapidly released fentanyl products and includes: Vomiting, nausea, constipation and drowsiness. Difficulty breathing should be immediately reported to a health care professional.
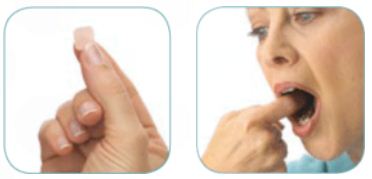
6. ONSOLIS - fentanyl buccal film
The FDA approved this unique dosage form of fentanyl in July of 2009. Onsolis, manufactured by MEDA pharmaceuticals, is an orally dissolving film containing fentanyl intended only for breakthrough cancer pain in patients 18 years of age or older. As with other fast-acting fentanyl products, Onsolis may only be used by patients already tolerant to opioids and also (like others) requires enrollment in their REMS program to prescribe, dispense and receive this drug. The REMS program for Onsolis is called "Focus" and this program will arrange for the direct delivery of this medication to the patient from a specialty pharmacy.
Onsolis is available in 5 different strengths:
- 200mcg
- 400mcg
- 600mcg
- 800mcg
- 1200mcg
How to use Onsolis:
Onsolis is used by wetting the inside of your cheek (with your tongue, or with a rinse of water). Carefully place 1 Onsolis film inside your mouth and press against the moistened cheek for 5 seconds. Onsolis will slowly dissolve in 15-30 minutes. Do not use more than 1 dose per episode of breakthrough pain, and do not repeat dosages sooner than 2 hours (max of 4 doses per day).
Side Effects: Nausea, vomiting, dizziness, dehydration and drowsiness. Difficulty breathing should be immediately reported to a health care professional.