Chemical Elements: Hydrogen and Calcium
Hydrogen
Symbol: H
Origin: Named by Lavoisier which comes from the word "hydro" and "gen" which means water forming.
Discovery: Hydrogen was discovered by Henry Cavendish of Britain in 1776.
Properties: It is the most abundant element in the universe and is the lightest too. It can be obtained from mines, oil, gas wells.
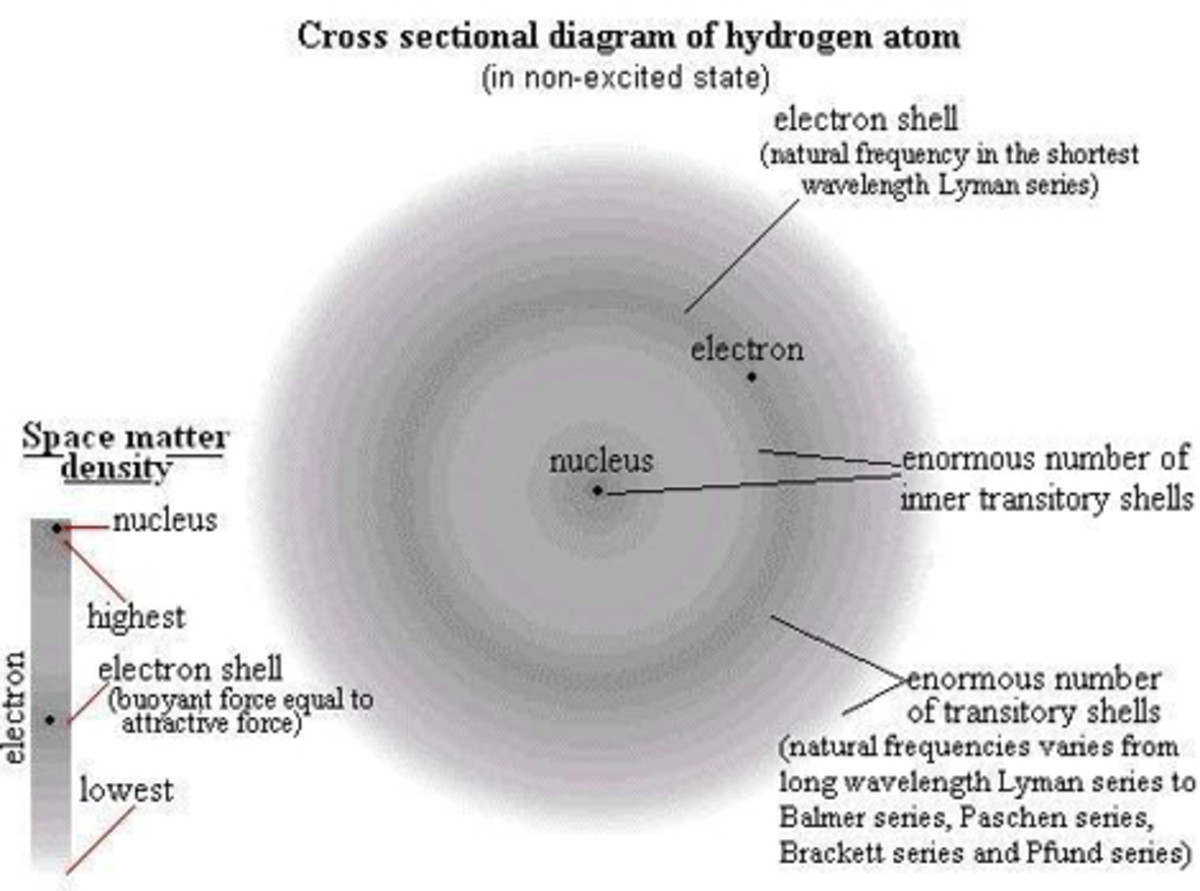
Hydrogen has the simplest structure. Hydrogen as water (H2O) is very much essential to life and it is present in all organic compounds. The thermonuclear fusion of hydrogen nuclei lights and heats the universe.
Hydrogen is the primary component of Jupiter and the other gas giant planets. Stars are made primarily of hydrogen. Also, the sun is a giant ball of hydrogen and helium gases. The process called fusion in the sun's core where hydrogen atoms combine to form helium atoms gives off radiant energy. This radiant energy sustains life on earth. The two most common methods for producing hydrogen are steam reforming and electrolysis (water splitting). Steam reforming is currently the least expensive method of producing hydrogen. It is used in industries to separate hydrogen atoms from carbon atoms in methane. Electrolysis is a process that splits hydrogen from water that is currently a very expensive process.
Uses: Rocket fuel, luminous paints, balloons, metal refining and trash converter.

Photo from ZeroAsterisk.com

Photo from Grassrootsstore.com
Calcium
Symbol: Ca
Origin: From the word "al" or lime.
Discovery: The British Chemist Sir Humphry Davy isolated calcium in 1808 by means of electrolysis.
Properties: Calcium is ductile and malleable metal when exposed to the air rapidly tarnish to yellow. It reacts violently with water forming the hydroxide and releasing hydride. It is essential for bone and teeth development.
Uses: Plaster of Paris, deodorizer, batteries, Portland cement, medicine.