Maximizing Copepod Aquaculture in the Presence of Ciliate Contamination through Temperature Control
Introduction:
Copepods are not only an important natural food source for numerous juvenile and larval marine fish species, but are often the preferred live food for rearing carnivorous and omnivorous larval fish in an aquaculture setting as well (Ajiboye et al. 2011, Santhanam & Perumal 2005, Shields et al. 1999). This can be attributed to a number of factors that contribute to making them an ideal food source. For one, their movement triggers a natural feeding response, which is important when feeding picky species or species that will not accept dried foods. The use of dried foods in place of live foods has even been shown to have a negative correlation to the growth and development of certain first-feeding larvae (Shields et al. 1999). Secondly, they have multiple life stages, each with different body-sizes, allowing for a wide range of feeding possibilities (Brown et al. 2003). The nauplii can be used to feed smaller, first-feeding stage larvae, while the adult copepods can be used to feed larger, later-stage larvae. Also, since they are indeed the natural diet of many larvae, they offer the benefit of being more readily accepted, and providing more adequate nutrition. Copepods fed an adequate diet are rich in highly unsaturated fatty acids including Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA), which are important for larval development (Ajiboye 2011, Shields et al. 1999). In comparison to other commonly fed zooplankton, copepods have superior nutritional content (Drillet et al. 2011, Stottrup 2000). Zooplankton species such as rotifers and Artemia sp. are more often the food provided for marine fish larvae; but unlike copepods they alone are considered nutritionally inadequate and must be enriched with additional nutrients before being used as feeds (Stottrup 2000). The prominence of these other zooplankton in the aquaculture trade can be attributed mostly to their ease of production in comparison to copepods (Stottrup 2000). Despite the significant benefits of using copepods as a feed, they remain underused due to the problems associated with culturing them (Santhanam & Perumal 2005).
Copepod production is made especially difficult by the combination of the limitations of maximum densities and the competitive interaction between copepods and nuisance ciliates (Ajiboye 20011, Drillet et al. 2011). These ciliates regularly appear in copepod cultures and can have severe negative influence on the sustainability of a copepod culture by means of competition for food, space, and possibly the consumption of copepod eggs (Drillet et al. 2011). This interaction likely decreases the maximum achievable density of copepods. The presence of these ciliates, if left unchecked, ultimately results in a culture crash and thus a loss in time, resources, and potential profit. Since copepods can only be grown very limiting quantities already, the effect of a culture crash is that much more detrimental to an aquaculture facility that depends on the constant availability of feeds. The production costs are directly related to possible culture densities (Drillet et al. 2011).
Our study will attempt to accomplish three goals: maximize copepod production, minimize the presence of nuisance ciliate contamination, and decrease the losses associated with the contamination. To do this, the study will focus on determining a temperature at which copepod growth is relatively high and ciliate growth becomes inhibited. Growth will be measured by percent change in concentration from the start to the end of the experiment. Cultures containing both copepods and ciliates and cultures containing just copepods (control) will be raised at various temperatures and the change in concentration of each organism assessed. Our hypothesis is that copepods will reach the higher densities in the presence of nuisance ciliates at ambient temperatures and at 22⁰C than at 26⁰C or 30⁰C.
Materials and Methods:
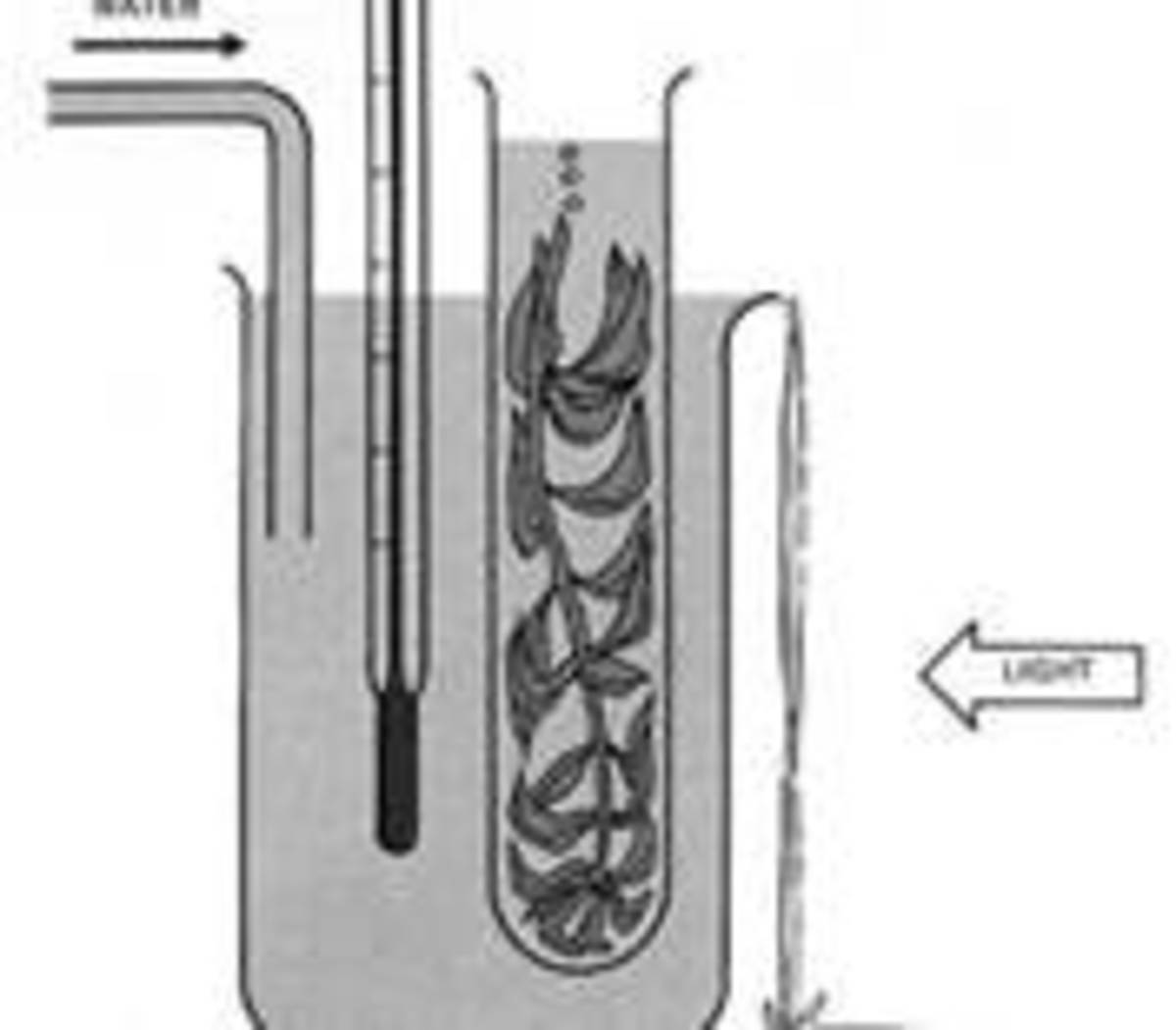
Eight water baths were constructed out of standard 37.85L glass aquariums, each containing 18L of fresh water, with two baths at each of the following temperatures: 22°C, 26°C, 30°C, and ambient (21°C +/- 1°). Each bath contained four plastic, lidded, one-liter cups with 800mL of 2μm filtered, pasteurized, 32‰ seawater in each. Aeration was administered to each container with airline hosing introduced through a small hole in the lid at the rate of one bubble per second. Each container was first inoculated with 2mL of Thalassiosira isochrysis at a density of 50,000cells/mL. Then, each container was inoculated with 27mL of water containing an average of 19 individuals of Parvocalanus sp.. Two containers were then chosen arbitrarily from each bath to be inoculated with 27mL of water containing an average of 399 ciliates and 53 individuals of Parvocalanus sp..
All containers were inoculated with 2mL of T. isochrysis at a density of 50,000cells/mL every other day. The experiment was maintained for a period of seven days, at which time samples were gathered. Three samples were collected from each cup in 27mL plastic cuvettes by temporarily increasing aeration to induce mixing while scooping underneath the water’s surface. They were then stained with Lugol’s iodine solution and preserved in a refrigerator at 4°C until counted. Samples were counted visually under a dissecting microscope and a plankton wheel.
Results:
Across all temperature treatments both copepod and ciliate percent change in concentration increased as temperature increased (figure 1). This was the case in both the controls and the copepods with ciliates. Ciliate concentrations actually decreased from the starting concentrations in the ambient (-2.77%) and 22°C treatments (-6.61%), whereas they increased in all other treatments.
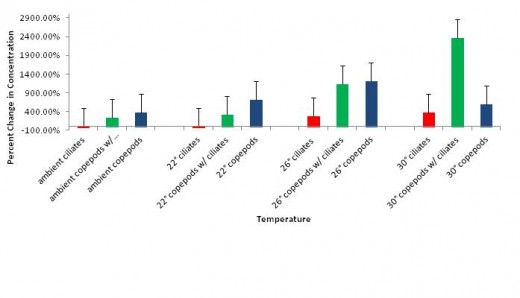
Figure 2 displays percent change in concentration of ciliates and copepods in the presence of ciliates. There is no apparent negative correlation between growth of copepods and the presence of ciliates alone. Copepods showed the greatest growth in the presence of ciliates at 30°C (2368.07% increase in concentration) and the least growth in the presence of ciliates at ambient temperatures (230.46% increase in concentration).
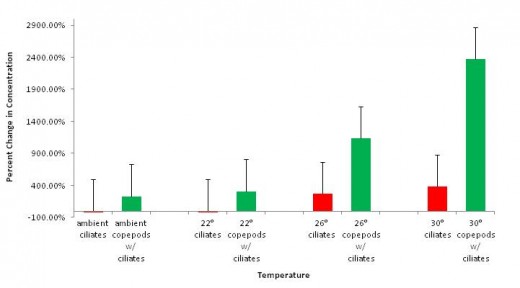
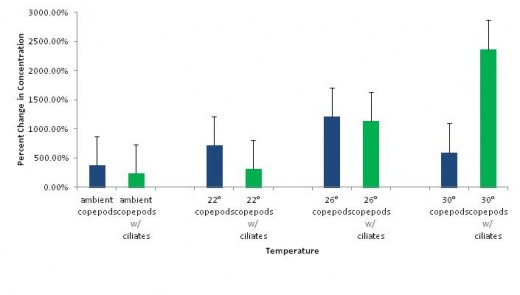
Figure 3 offers a comparison of percent change in concentration of control copepods against copepods in the presence of ciliates. Copepods experienced greater growth in the absence of ciliates than in the presence of them in all treatments with the exception of 30°C. Copepods grown in the presence of ciliates exhibited a 2368.07% change in concentration, the highest of all treatments. Copepods grown alone at 30°C exhibited a comparatively smaller increase in concentration of 591.08%.
Discussion:
Our hypothesis was supported by the results identified in this study. As shown in figure 1, the concentrations of ciliates in both ambient and 22°C treatments decreased from start to finish, while the concentrations of copepods increased at these same temperatures. This implies that these lower temperatures do indeed inhibit the growth of ciliates, without an apparent significantly inhibiting the growth of the copepods. Growth of both the ciliates and copepods was positively correlated with an increase in temperatures (figure 1), and maximum densities of both organisms were achieved at 30°C when grown together. Despite this, we still suggest that copepods be grown at ambient temperatures or at 22°C in order to minimize the possibility of a ciliate-induced culture crash. The decrease in ciliate concentration at these temperatures could imply a die-off, which is not apparent in any of the higher-temperature treatments. If a die-off of ciliates was the case, this would provide an ideal temperature for minimizing losses due to culture crashes. Since both low-temperature treatments resulted in negative percent change in concentration of ciliates, ambient temperature is likely the most cost-effective option as it would reduce costs associated with energy consumption from heating.
It is interesting to note that copepods had a higher percent change in concentration when grown in the absence of ciliates than when grown with ciliates in all temperature treatments with the exception of 30°C. At this temperature, copepods reached higher densities in the presence of ciliates. This could be attributed to sampling errors, or to the fact that there were more copepods present at the starting point in the treatments with ciliates. Another possibility to consider is that increasing temperature can have a positive effect on algal growth, causing an increase in availability of food for the copepods and ciliates (Goldman & Carpenter 1974, Raven & Geider 1988). This could possibly decrease the amount of competition and allow both species to coexist, though the likelihood of this relationship is questionable and as thus is grounds for further study.
Surprisingly, in the absence of ciliates, copepods experienced the most growth in 26°C treatments, as opposed to at 30°C in the presence of ciliates (figure 2). This may also be attributed to sampling error, though it is be possible that there is an unidentified copepod-ciliate interaction at higher temperatures that contributes to increased growth of copepods in the presence of ciliates. This too is grounds for further study. Other studies have found that increased temperature is correlated with a decreased time to maturity (Klein Breteler et al. 1995, Rhyne et al. 2009). This could contribute to high positive changes in concentration because organisms become sexually mature faster and can thus reproduce more in a given time frame. Studies have also found that optimum growth rates of copepods in culture occur between 25°C and 30°C (Rhyne et al. 2009).
In future trials of this study, it would be advisable to introduce the same amount of copepods to all treatments in order to achieve more reliable results, and to apply a sampling method that more accurately represents the changes in concentrations. Counting the entirety of each container, though time consuming, would likely prove much more accurate. In reference to our data, we would suggest that copepods be grown at ambient temperatures (21°C +/- 1°) in order to minimize and even impede ciliate growth while still achieving sufficient copepod growth and minimizing losses associated with energy consumption and culture crashes. Growing copepods at 30°C in the presence of ciliates did yield the highest concentrations, but also the highest yield of ciliates. If the culture crashes could be predicted, there may be applications for having ciliates present in order to achieve higher growth.
Acknowledgements:
Special thanks to Dr. Andrew Rhyne and Brad Bourke for their help in the design and construction of the experiment
Literature Cited:
Ajiboye, O. O., Yakubu, A. F., Adams, T. E., Olaji, E. D., & Nwogu, N. A. 2011. A review of the use of copepods in marine fish larviculture. Reviews in Fish Biology and Fisheries. 21: 225-246.
Brown, R. J., Rundle, S. D., Hutchinson, T. H., Williams, T. D. & Jones, M. B. 2003. A copepod life-cycle test and growth model for interpreting the effects of lindane. Aquatic Toxicology 63:1-11.
Drillet, G., Frouël, S., Sichlau, M. H., Jepsen, P. M., Højgaard, J. K., Joarder, A. K. & Hansen, B. W. 2011. Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture 315:155-66.
Goldman, J. & Carpenter, E. 1974. A kinetic approach to the effect of temperature on algal growth. Limnol. Oceanogr. 19:756-766.
Klein Breteler, W. C. M., Gonzalez, S. R., Schogt, N. 1995. Development of Pseudocalanus elongatus (Copepoda, Calanoida) cultured at different temperature and food conditions. Marine Ecology Progress Series. 119: 99-110.
Raven, J. & Geider, R. 1988. Temperature and algal growth. New Phytol. 110:441-61.
Rhyne, A. L., Ohs, C. L. & Stenn, E. 2009. Effects of temperature on reproduction and survival of the calanoid copepod pseudodiaptomus pelagicus. Aquaculture 292:53-9.
Santhanam, P. & Perumal, P. 2005. Marine copepods - the living capsules. Seshaiyana 13:6-8.
Shields, R. J., Bell, J. G., Luizi, F. S., Gara, B. 1999. Natural copepods are superior to enriched Artemia nauplii as feed for halibut larvae (hippoglossus hippoglossus) in terms of survival, pigmentation and retinal.. J. Nutr. 129:1186-94.
Stottrup, J. 2000. The elusive copepods: Their production and suitability in marine aquaculture. Aquacult. Res. 31:703-11.