Hydrogen Cell Technology

What Is A Hydrogen Fuel Cell?
A hydrogen fuel cell uses hydrogen in a chemical reaction to generate electricity. The reason this form of power generation is a much better alternative to burning fossil fuels is because the only by-product or exhaust leftover from the reaction is water and a little heat.
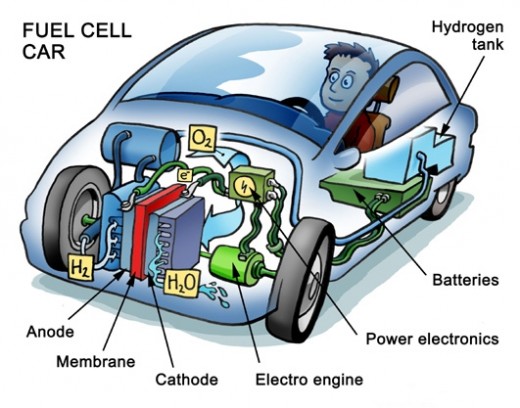
Much like a battery this fuel cell uses two electrodes, an anode (negative electrode) and a cathode (positive electrode). But that is where the comparison to a conventional battery ends. The method by which electricity is created from the reaction in the fuel cell is shown in the diagram below.
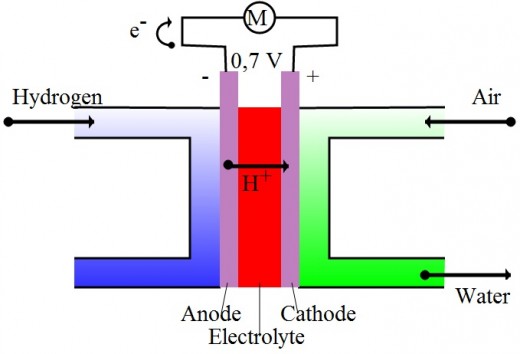
The Fuel Cell Process
1) Hydrogen is introduced to the Anode where the electrons are stripped from the hydrogen atoms and become electricity.
2) The left over positively charged hydrogen ions (with no electrons) pass through the electrolyte and reach the cathode.
3) At the cathode air is introduced and the oxygen from the air readily bonds with the leftover hydrogen ions to form water.
Incidentally the process also works in reverse when electricity is passed through water it can also create hydrogen and oxygen.

Practical Applications and Uses
Even now across the world there are hydrogen fueling stations and while it is true that they are a small fraction compared to gasoline fueling stations the amount continues to grow. Currently there are 85 hydrogen fueling stations in the United States.
The hydrogen fuel cell generates electricity which runs an electric motor in the vehicle. So far manufacturers have developed automobiles, buses, forklifts, motorcycles, airplanes, boats and submarines which use hydrogen fuel cells for operation. Other than motor vehicles this technology is also being incorporated into telecommunications equipment, emergency power generation, and even electronics such as laptops, cell phones, and small appliances.
PROS
1) It's non-destructive to the environment. Water is converted into hydrogen and oxygen, the hydrogen goes through the fuel cell creating electricity and then recombines with oxygen to return to water. It is a continuous cycle and leaves no waste that could destroy the environment.
2) Home fueling devices can be use solar power to perform electrolysis of water as mentioned earlier to separate the hydrogen from the oxygen.
3) To the consumer this means that you can create the fuel that powers your vehicle at no cost. Fueling stations also use this method becoming self sufficient.
CONS
1) As a new technology the demand for hydrogen from fueling stations is still low thus causing such companies promoting the technology financial instability.
2) Some parts of the cell can be very expensive and this drives up the price of using the technology.
3) Substandard materials used in fuel cells can be more susceptible to corrosion.
4) The fuel cell can be sensitive to both high temperatures and impurities in the fuel such as carbon dioxide.
Hydrogen Fuel VS. Gasoline
Overall the continued use of fossil fuels is unsustainable which is not just due to limited supply but also the detrimental effects to the environment. As of 2010 the amount of vehicles has surpassed the one billion mark and the vast majority of these run exclusively on fossil fuels. One billion trails of black smoke spewing from each machine, let alone industrial manufacturing stacks, and other forms of pollution. In contrast if these one billion vehicles ran on hydrogen fuel cells they would be creating zero pollution.