Chikungunya Virus
Chikungunya (CHIKV) is an arborous that belongs to the Alpha virus genus under the family Togaviridae. The term ‘Chikungunya’ originated from the Kimakonde language, which means ‘to be contorted or bent’. Chikungunya is a viral disease that is transmitted to the human blood stream through infected mosquitoes. The most significant symptom is pain in the joints, causing the patient to become weak. Other symptoms include high body temperature, swelling in the joints, body rashes, headaches, fatigue and nausea. In 1952 it was found in various regions of Asia, Africa and parts of Southern Europe. In this essay, we will discuss about the structure and mechanism of interaction between the CHIKV virus and human cells.
Transmission
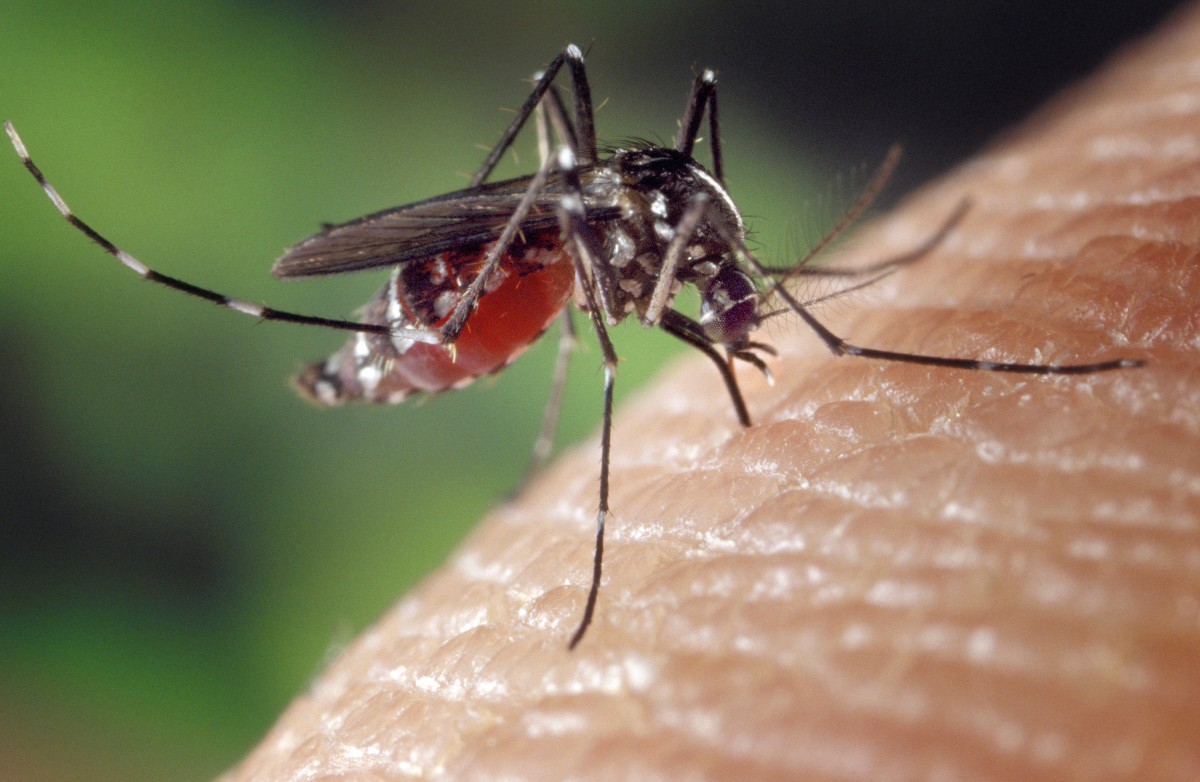
Chikunguya virus is usually transmitted from one human to the other through mosquito bites that have been infected. In most cases, mosquitoes that are involved in this transmission are the aedes albopictus and aedes aegypti. These kinds of mosquitoes usually bite through the day hours. The peak of their activities happens to be morning hours and the late afternoon. Both the aedes albopictus and aedes aegypti species bite outdoors, but aedes aegypti can also feed when indoors. In particular, these viruses are transmitted through bites from infected aedes mosquitoes, which acquire the virus when feeding on an infected person’s blood. After the virus’ incubation period, which takes approximately, five to ten days, the mosquito is capable by blood feeding and probing, to transmit Chikungunya virus for as long as it lives. However, there is no particular way of telling if a specific mosquito has this virus (Martin, 2007).
Female mosquitoes are also capable of transmitting the virus to their offspring through transovarial transmission. However, the role that this plays in sustaining the virus transmission to human has not yet been established. Infected human beings are the main multipliers and carriers of the virus and they serve as a source for mosquitoes, which are uninfected. The virus circulates inside the infected human blood for several days, just about the same time that they experience Chikungunya fever. Aedes mosquito can acquire the virus if they bite an infected person during such periods. In some parts of Africa and Asia, the circle of transmission may involve primates of the jungle who may act as reservoirs for the virus. Further, Aedes mosquitoes usually prefer breeding in water-filled receptacles, mainly close to habitation of human. They rest in dark rooms such as under beds and bathrooms and breed in the small pools that harbor superfluous human waste (Enserink, 2007).
Another way through which Chikungunya fever is transmitted though rarely is from mother to child. In this respect, the virus may be transmitted from the mother to the child during the time of delivery. According to Ross (2008) chikungunya infection from the mother to child may occur in the case of intrapartum maternal viremia, consequently leading to extreme neonatal inflection. In essence, the virus represents a substantial risk especially for neonates who are born to viremic parturients that should be considered by healthcare practitioners in the case of chikungunya epidemic. Ross goes on to add that transmission of chikungunya is rare at the level of the population expect in antepartum.
However, the virus appears to be common or persistent in the context of maternal viremia. In this situation, the virus is usually related with extreme neonatal complications. Given the authors observation that mother to child infection particularly occurs in the context of maternal viremia that is common during delivery and that delivery through C-section may not prevent CHIKV transmission, the author advices a close monitoring of viremic parturients and ensure that they deliver in maternal facilities that harbor sufficient neonatal and obstetric care (Ross, 2008).
In general, it should be considered that mother to child transmission of CHIKV occurs only at rare instances and mostly prior to 22 weeks of gestation. However, if this occurs, it becomes potentially dangerous to both the mother and the child. In addition, there is also a possibility of transplacental transmission, though the pathologenic mechanism is not yet established.
The third way in which CHIKV is transmitted is through infected blood. In this perspective, it has been theorized that blood transfusion is one of the ways of transmitting CHIKV virus from an infected one to uninfected one, though there no proof yet.
Symptoms
The incubation period for this virus is two to six days with its symptoms generally appearing four to seven days. Infected people will notice several symptoms some of which are: Rash, joint pain, lower back pain, nausea chills, headaches and fever. Upon infection, the virus tends to show itself in two different phases (Schilte,et al 2013).
a) The Acute phase
This phase typically lasts from few days to some weeks with characteristics ranging from abrupt chills onset, high fever, nausea, vomiting, headache, joint pain, and on occasions maculopapular rashes characterized by sported and raised lesions. The main and most severe symptom in this phase is muscular pain. This pain tends to be extremely intense such that it prostrates its victims. This fever typically lasts approximately two days after which it disappears abruptly. Other symptoms such as insomnia, headache, and extreme prostration degree, may last up to seven days (Powers and Logue, 2007).
b)The chronic Phase
This phase is chiefly characterized by poly-arthralgia, which can last for weeks to years. Patients in this stage generally suffer from joint pain for a period that can last up to two years, depending on their age. More than 95 percent of adults who are infected are only symptomatic after infection, many of who become disabled for either weeks or months due to decreased dexterity, delayed reaction and loss of mobility. Recurrent pain in the joint is experienced by about 40 percent of the infected. Rare but very serious complications of this disease are noted during early epidemics .This includes, inflammation of heart muscles, meninges and brain, as well as haemorrhage. Other complications include retinitis and uveitis. More than half of chikungunya fever patients appears to be 65 years and above with 33 percent of them dying from the illness. Children are in some cases disproportionally affected by this fever (Powers and Logue, 2007).
Diagnosis
Infections from Chikungunya are mostly confirmed by detection of the virus, specific antibodies in patient’s samples, or viral RNA. In most cases, the type of test performed is determined by volume of available samples and timing. Blood test appears to be the most reliable way since the symptoms are similar to dengue fever. Other common tests include serological tests and RT-PCR (Ross, 2007).
RT-PCR
Viral RNA can easily be detected by reverse transcriptase-polymerase chain reaction (RT-PCR) inside serum specimens that are obtained from patients in the course of acute phase. A Chikungunya infection causes high viremia levels, which may typically last for 4-6 days following the onset of illness. This makes it possible for RT-PCR to be performed within 7 days on acute-phase specimen in order to confirm infection.
ELISA
Enzyme-linked immunosorbent essays (ELISA) can detect both lgG antibodies and anti-CHIKV immoglobulin from either convalescent-phase or acute samples. Serological diagnosis usually requires a large blood amount than other methods. The results of ELISA requires two to three days and the test itself involves very little cross reactivity with similar alphaviruses
PRNT
Plaque reduction neutralization tests (PRNT) are the most useful since they are specific for alphaviruses and the gold standard in confirming serologic test results (Lenschow, 2013). The only drawback to this method is that it normally requires the use of a live virus. However, PRNT should only be performed in Biosafety level 3 laboratories (BSL-3) since it requires specialized laboratory containment equipment.
Treatment
Currently, there has been no specific treatment nor vaccine that has been found for Chikungunya. In most cases, the virus is symptomatically treated, with fluids, bed rest, and medicine that relieve symptoms of aching and fever. Such medicine includes naproxen, ibuprofen, paracetamol, and acetaminophen. However, aspirin must be avoided at all cost. Nonetheless, individuals who are infected should be protected from further exposure to mosquito during the early days of the illness in order to prevent contribution to the transmission circle. There should be no cause for alarm since this fever can practically be cured by the immune system in most of the cases. In particular, chloroquine has gained recognition as the most preferred possible treatment for chikungunya-associated symptoms, and as an anti-inflammatory agent that can combat arthritis that is associated with Chikungunya virus (Powers and Logue 2007).
A study from Malaya University established that chloroquine phosphate has produced very promising results for arthritis-like symptoms, which are not relieved through aspirin and non-steroidal anti-inflammatory drugs (NSAID). However there have been many debates regarding the effectiveness of chloroquine as a treatment fo Chikungunya virus since some unpublished studies in monkeys and cell culture portrays no effect of treatment by chloroquine in reducing chikungunya virus infection. Further, homeopathic experts argue that effective drugs that speed up recovery and prevent infection are available. This type of treatment has been tried out in certain cities of south India and it has been claimed that Eupatorium perf actually prevents Chikungunya infection. Other type of medicine that have been prescribed for this disease include Rhus-tox, Pyroginum, Influenzinum, Cedron, Arnica, and China (Schuffenecker and Michault, 2006).
Prevention
There is currently no credible way or available anti-viral medications for preventing this virus. However, it has been proved that an individual’s immune system can adequately serve as the best fighter in defeating this virus. What is more, mosquito repellants are also good preventing measures since they protect one from mosquito bites. Furthermore, putting on clothes, which covers a person’s extremity, is also a great help. Another prevention measure is to eliminate breeding sites for mosquitoes. Practically, there are various ways to reduce mosquitoes, but the most effective is to eliminate places that act as laying ground for mosquitoes such as artificial containers, which hold water around the homestead. In urban centers, these mosquitoes breed on collections of water such as used tires, plastic bags, flowerpots, broken bottles and other objects that may hold water. Therefore, removing artificial containers or periodic draining is the most efficient way to reduce breeding grounds for mosquitoes (Enserink, 2007).
Larycide treatment is one of the proven methods in controlling vector larvae. However, the chosen laryicide should be preferable and long lasting. In addition, there are specific insect growth regulators (IGRs) which are available and are both long lasting and safe. For reduction of adult mosquito load, insecticide fogging is rather helpful. For the purpose of eliminating standing water, it is paramount to change birdbath water at least once a week, get rid of unwanted or old tires in the yard, empty wading pools for children at least weekly, and empty unused containers or hoard them upside down (Schilte, 2013).
Natural control
In 1998, some scientists in Vietnam and Australia’s ministry of health devised a scheme, which encouraged people to be placing the crustacean mesocyclops, a water bug, in discarded containers, and water tanks where Aedes mosquitoes were believed to thrive (Martin, 2007). This method has been viewed as more environmentally friendly and cost effective than others. However, the method requires a community’s continuous participation.
Conclusion
Symptoms of CHIKV may be observed from one to twelve days of conceiving the virus. As mentioned earlier, Chikungunya is rarely fatal and its symptoms can prolong for a few days to a few months, depending on the immune system of the infected human. The lipid envelope of the virus helps in detection of this virus. Currently, doctors have not yet found a medicine that effectively treat Chikunguny disorder, and therefore, we need to protect our family and ourselves from this virus.
References
Enserink M.( 2007). The Chinkungunya Virus. Science 318(5858):
1860–1. doi:10.1126/science.318.5858.1860.PMID 18096785
Martin E (2007). EPIDEMIOLOGY: Tropical Disease Follows Mosquitoes to
Europe. Science 317 (5844): 485.doi:10.1126/science.317.5844.1485a. PMID 17872417
Powers AM, Logue CH. ( 2007). Changing patterns of chikungunya virus: re-emergence of a
zoonotic arbovirus. J. Gen. Virol. 88 (Pt 9): 2363–77. doi:10.1099/vir.0.82858-0. PMID 17698645.
Ross RW (2007) . The Newala epidemic. III. The virus: Isolation, pathogenic properties and
relationship to the epidemic. J Hyg (London) ;54:177–191. [PMC free article] [PubMed].
Schilte, C; Staikowsky, F; Couderc, T; Madec, Y; Carpentier, F; Kassab, S; Albert, ML; Lecuit,
M; Michault, A (2013). Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study.". PLoS neglected tropical diseases 7 (3): e2137.
doi:10.1371/journal.pntd.0002137. PMID 23556021.
Schuffenecker I, Michault A. (2006). Genome microevolution of chikungunya viruses causing
the Indian Ocean outbreak. PLoS Med. 3 (7): e263. doi:10.1371/journal.pmed.0030263. PMC 1463904. PMID 16700631.