Carbohydrates - Simple Sugars

What Are Carbohydrates?
- Carbohydrates are a group of organic compounds (including sugars, starch and cellulose) that contain hydrogen, carbon and oxygen.
- The general formula for carbohydrates is Cn(H2O)n.
- Carbohydrates make up about 10% of the organic matter of a cell.
- Carbohydrates have a number of different functions; they act as an energy source (releasing glucose during respiration), they can also function as an energy store and provide structure for the cell (for example cellulose makes up plant cell walls).
- Some carbohydrates will form parts of larger molecules, for example nucleic acid.
- The number of carbon atoms is always the same as the number of oxygen atoms in a carbohydrate molecule.

Simple Sugars - Monosaccharides
The simplest carbohydrates are called monosaccharides which are the monomers of carbohydrates.
All of the larger carbohydrates are formed by the monosaccharides joining together (these are called disaccharides).
There are a number of different monosaccharides which contain between 3 and 6 carbon atoms.
The properties that these monosaccharides possess are all very similar.
The properties are:
- They are all soluble in water.
- They all form crystals.
- They are all sweet tasting.
The different monosaccharides are grouped according to the amount of carbon atoms that the molecule contains.
- When a monosaccharide molecule has 3 carbon atoms they are known as triose sugars.
- When they have 5 carbon atoms they are known as pentose sugars.
- When they have 6 carbon atoms they are known as hexose sugars.
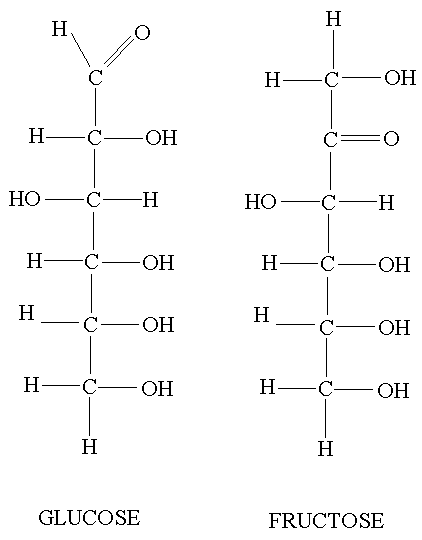
Hexoses are the most common monosaccharide molecule, these include glucose and fructose.
Glucose and fructose molecules have the formula C6H12O6.

Glucose
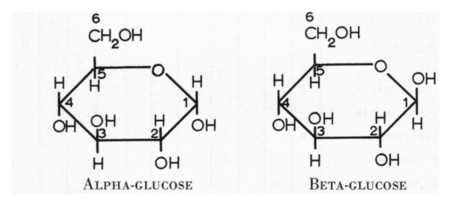
- Glucose can be drawn as a chain or a ring structure.
- When the ring structure forms it can form in 2 different ways.
- The H and the OH at carbon 1 of the ring can be above or below the plane of the ring.
- The two different types of glucose are called alpha glucose and beta glucose.
- In alpha glucose the OH at Carbon 1 is below the plane of the ring whereas in beta glucose it is above the plane of the ring.
- The two different forms of glucose are isomers of each other.
- The difference in structure leads to different properties.

Monosaccharides and Disaccharides
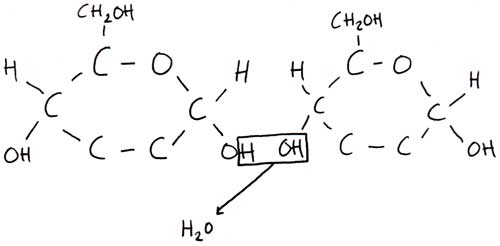
- Two monosaccharide molecules can join together in a condensation reaction, this forms a disaccharide molecule.
- The OH from one monosaccharide molecule and the H from the other monosaccharide molecule form H20 and is eliminated.
- The bond formed between the disaccharide molecule is covalent and is called a glycosidic bond.
- The reverse is when you add water to a disaccharide molecule and the glycosidic bond breaks down, this is called a hydrolysis reaction.
- Lots of monosaccharide molecules can join to form polysaccharide molecules.