Acid Rain—Cause, Effect, Solution


Acid rain--old fiend
For the past 165 years since discovered, the knowledge toward acid rain had been in- creasing. The relationship between acid rain and industrial pollution is also noticed widely throughout the world. However, can our knowledge be used to fight against acid rain? Or, more likely, how can we minimize the chances that this phenomenon may occur?
Since 1853, people became aware of the occurrence. But it wasn’t until the late 1960s did scientists started studying it. Even the term “acid rain” was unknown until chemist Robert Angus Smith coined it in 1872.
Also called acid precipitation, acid rain is “rainfall made sufficiently acidic by atmos- pheric pollution that it causes environmental harm”. Rainwater with a pH reading lower than 5.5 is considered acid rain. It is especially damaging to forests and lakes. While a small portion of acid precipitation is created by natural sources like volcanoes, most are created by heavy vehicles, burning of fossil fuels, manufacturing, oil refineries, etc.
What, how and where?
There are 3 main chemical compounds that are the culprits of acid rain—SO2, SO3, and NO2. Sulfur dioxide, a toxic gas, is produced mainly by volcanic activities, while sulfur trioxide is a byproduct of burning fossil fuels. Nitrogen dioxide is a brownish red gas that is released in the measure of tons each year by cars, power-plants, etc. All three compounds react easily with water, thereby creating acid rain.
Sulfur dioxide combines with water to form H2SO3. The chemical equation for this is: SO2+H2O→H2SO3. Named sulfurous acid, it is the intermediate stage of the formation of acid rain. The volcano releases great amounts of SO2 into the atmosphere. Then the compound reacts with water droplets in clouds, for instance. Sulfur dioxide is very reactive with water, making it one of the key gases for creating acid rain.
The key difference between SO3 and SO2 is the number of oxygen atoms bonded to the sulfur atom. Also, SO3 is solid while SO2 is a gas. SO3 is mainly created artificially because it requires intense heat. Therefore, SO3 is mainly the byproduct of fossil fuel burning. Sulfur triox- ide dissolves in water to form a liquid solution called sulfuric acid. In a case in which enough SO3 is added and the solution is vaporized, gaseous state sulfuric acid is formed. Once it is re- leased and carried out by wind, the SO3 reacts with H2O to form H2SO4, or sulfuric acid. Chemi- cal equation for this reaction is: SO3+H2O→H2SO4. Though a high amount of sulfuric acid is released, the majority of acid rain is composed of only a miniature amount of SO3. Sulfuric acid is slightly more acidic than sulfurous acid.
In nature, most nitrogen oxides are produced in a lightning. The high temperature causes oxygen and nitrogen to react, forming NO, or nitrogen monoxide. Chemical equation for that re- action is: N2+O2→NO. NO then reacts with oxygen once more, forming NO2, or nitrogen diox- ide. Chemical equation: 2NO+O2→2NO2. The two compounds are collectively known as NOx, or nitrogen oxides. As mentioned earlier, natural sources of NOx include lightening. Human sources include combusting fossil fuels. Nitrogen dioxide reacts with water to form two prod- ucts—nitric acid and nitrogen monoxide, based on equation: 3NO2+H2O→2HNO3+NO. HNO3, better known a nitric acid, is a strong, highly corruptive acid. We can also summarize the reaction as: NOx+H2O→HNO3.


There are controversy in whether carbonic acid, or H2CO3, creates acid rain. It is slightly acidic, but only to the range 5.6 on the pH scale. Long-term exposure to carbonic acid can cause damage. However, it is relatively weaker than any of the ones mentioned above.
Acid rain does damage in multiple ways: aquatic environment, forestal environment, soil environment, and on public health.
Acid rain can either fall directly into bodies of water or flow into rivers and streams. Most river organisms live under a pH scale of around 4.8. However, the adding of acid rain to bodies of water lowers the overall pH, thereby making it unsuitable for life. Snails, mussels, and clams, which need a higher pH level, is specially vulnerable in the presence of acid rain. By reducing biodiversity, water ecosystems are broken and people would be facing uncontrolled flour- ishing or extinction of species. Similar problem are being faced in forests and woods. Especially in Germany, Poland, and Switzerland, trees are made vulnerable to diseases while insects face starvation since leaves are destroyed.
Similar to water, soil is also made acidic by acid rain. Microorganisms cannot adapt to such a great change in pH, so they dies. Hydrogen atoms in the acid rain would also destroy vital minerals helpful for farming, like calcium. Aluminum is “allowed to come into solution”2, and since it is toxic to plant roots in its organic form, it can break plants and prevent them from absorbing nutrients.
Solutions
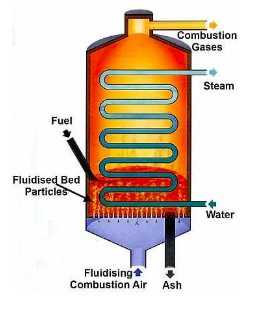
There are no such things as stopping acid rain in mid-air, but we can reduce the amount of SO2 and NOx released so that there are less acid rain. One way is by using renewable resources like solar energy and hydroelectric energy. But that’s not the only way. Newer technologies capture the chemicals before they get exhaled. One example is fluidized bed combustion, or FBC. In this combustion system, fossil fuels are burned, but a fluidized bed—a bed of limestone, in this case—reacts with the sulfuric acid and produces water, carbon dioxide, and calcium sulfate, or CaSO4.
Limestone is the crystal form of calcium carbonate. When limestone reacts with acid, it breaks down and dissolves. In other words, H2SO4 reacts with limestone before getting released. The original acid turns to water in this process (CaCO3+H2SO4→H2O+CO2+CaSO4) because H2CO3→H2O+CO2. This way, more than 90% of H2SO4 is converted, resulting in less acid rain.
By using a suitable fluidizing bed, we can reduce waste and toxic gas by a great amount. In the reaction, the acid turns to water, which of course, is no harm to the environment. Also, the combustions happen at approximately 750-900°C, therefore largely reducing nitrogen oxide emissions, which form at about 1500°C. As you now see, fluidizing bed combustions play a predominately part in reducing the two main gases blameworthy for acid rain formation. However, this technology has drawbacks. As I’ve mentioned before, the reaction releases carbon dioxide, which is the main cause of global climate change. Too much CO2 in the atmosphere would trap the heat inside Earth, leading to global climate increase. Also, to use this combustion system, a large amount of water is required to be boiled, thus raising the need for energy. To boil all the water requires huge amount of time, reaching up to 48 hours in some cases. That makes it insufficient. Fluidized bed combustion can be expensive to fix, for the great amount of waste gas that pass through the vents cause erosion. All in all, FBCs can reduce toxic gases such as SO2 and NOx, which are the main causes of acid rain; however, it can create CO2, which hurt the environment not by making it toxic, but raising the heat trapped inside and hurting the atmosphere.
There may not be a perfect solution, but remember--things as simple as telling someone you know that drives to lessen the miles works as well! Take the metro; Ride a bike if you like! Acid rain wouldn’t be going away the next year, but by doing all the small things in you life, you are helping everyone to fight against it.