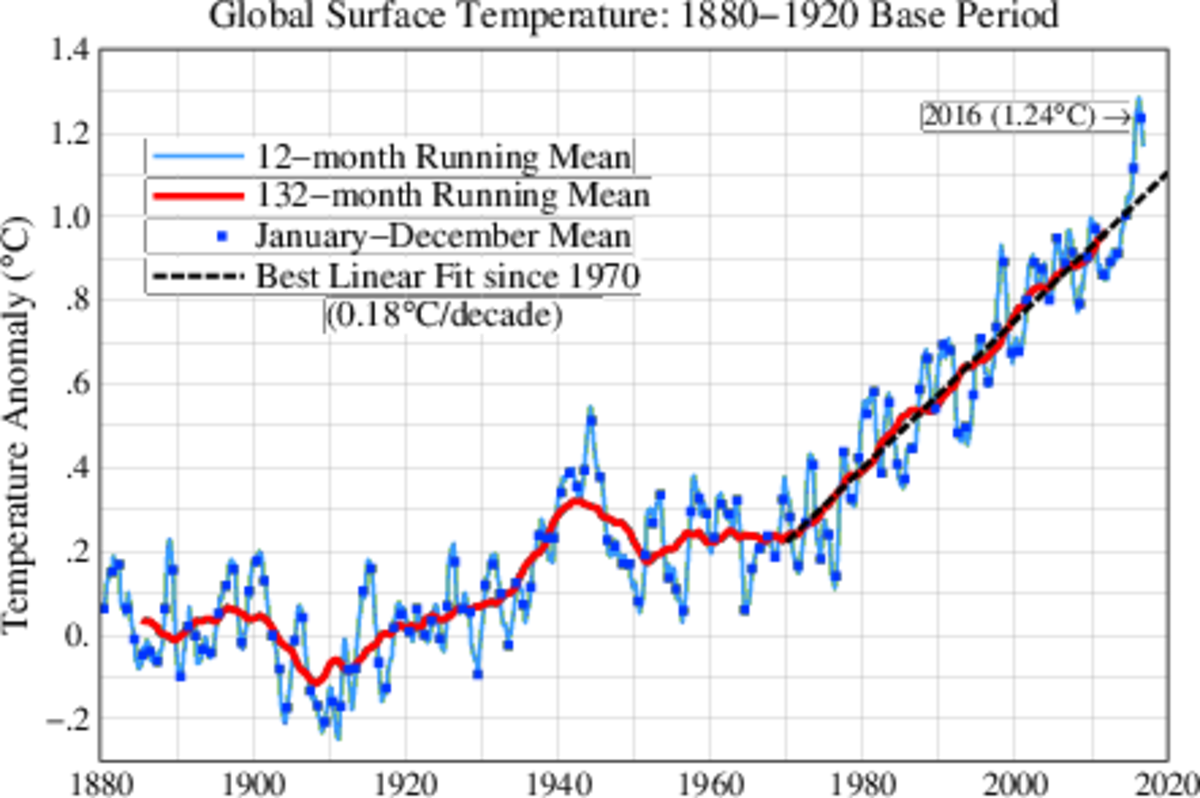
Alternative Biochemistries Available to Life
Scientists Discover Arsenic Fulfilling the Role of Phosphorus
All life on Earth needs carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These are the elements which all known life, ever discovered, always contains. But now, NASA researchers have discovered a type of bacteria that lives in a lake, here on Earth, that seems to use arsenic, which is typically poisonous to most lifeforms, to construct it's DNA sugar backbone (usually phosphorus is used here). Additionally, the arsenic is also replacing the phosphorus role of building the ATP molecule (adenosine triphosphate). This is a major peculiarity. As different as life appears to be macroscopically, in the inner details everything is almost exactly the same, at least chemically. But now, this discovery shows, for the first time, a major chemical structure difference. The information coding polymer inside these strange cells are still DNA, leading some experts to believe this life did not develop on it's own separate lineage. It probably is not alien, instead it is a significant and novel mutation / adaptation. Arsenic is directly under phosphorus on the period table of elements, meaning these two elements share some electronic configuration properties, allowing similar types of bonds to form. Still, despite the similarities, the differences are usually more apparent. Hydrogen sulfide for example, is a gas, smells like rotten eggs, and is very poisonous, but is structurally similar to water, having only the oxygen of H2O swapped with sulfur. Like the situation with arsenic, sulfur is directly below oxygen on the periodic table.
There has been some doubt as to whether this lifeform found in California (of all places) was influenced artificially to use arsenic instead of phosphorus by human induced natural selection. Regardless if it is natural or not, the point is that it can in fact support life. This finding is probably the single biggest clue ever, that alternative biochemistries are more than a theoretical curiosity. They can, at least occasionally, occur.

Silicon Based Lifeforms
Silicon based life is the most common hypothetical alternative to life in science fiction. However, there is also a scientific basis to this idea. Silicon, being in the same group as carbon, on the periodic table, shares carbon's ability to form a limitless number of molecules. This diversity would give life a method of encoding biological information just like the amino acids based on carbon in real life.
Silicon has it's drawbacks though. For one, it's bond strength is higher than that of carbon's and tends to make rigid structures rather than the flexible gooey materials that life as we know it consists of. Take silicon dioxide for example, this is analogous to carbon dioxide in Earth life. Assuming all other details were identical, a silicon life form would breathe in oxygen and breathe out silicon dioxide, similar to how humans and animals breathe out carbon dioxide. The only problem is that silicon dioxide is sand. This would not flow well out of a set of lungs!
Maybe, on planets with higher temperatures, liquid silicon would behave more organically analogous to carbon on Earth. Then the exhalation of such beings would be a vaporized rock, which just might work.
Still, there are other difficulties, like water for example. At the temperatures where silicon just might allow enough flexibility for the necessary internal movements of life processes, water will no longer exist, having long ago evaporated and disassociated into hydrogen and oxygen. At high atmospheric pressures, like on Venus - but probably higher, water might persist.
Interestingly, silicon is very abundant on Earth and carbon is, relative to silicon, very rare. But, like is carbon based here. This seems to suggest that carbon is the more likely way to build life on Earth like planets. In other words, if silicon COULD work, then it WOULD have. This only applies to Earth, of course. Alien environments might prefer silicon.

Replacing Water with Ammonia
Discovering alien life which doesn't use water would revolutionize the definitions of life. Many biologists still blindly follow their teachings that water is the number one thing all life will have in common. Chemists and physicist know, however, that ultimatums like these never hold. With a vastly different chemical system, in a vastly different environment, life could arise without the need for water. In fact, water might be poisonous to these organisms. Ammonia for example, has many chemical similarities with water, some of which are superior is certain ways, making ammonia the number one favorite in alternative hypothetical biochemistries .
For us water derived creatures, here on Earth, ammonia is pretty poisonous, we can tolerate it in certain ways, but typically try to eliminate it from our body, such as through urination. An ammonia solvent based organism, might have these feelings toward water, barely being able to tolerate it, but realize it is so close to being acceptable, but not quite.
Ammonia has different temperature ranges from water, and is a gas at room temperature at standard pressure. At cold temperatures, like in Jupiter's atmosphere or moons, or Saturn, ammonia would exist as a liquid phase over a wide range of variables. On a planet with liquid ammonia lakes and oceans, the clouds would be made of ice crystals of ammonia, and the rain would be liquid ammonia.

Forcing Evolution
An interesting experiment some time ago, involved artificially selecting yeast cells to tolerate higher temperatures. The yeasts' habitat (tank) temperature was slowly raised over several months. The very gradual temperature increases caused miniature extinctions to occur within the population, however there were always survivors. These survivors, through reproduction, eventually repopulated the system fully, but were completely tolerant of the now higher temperature. This cycle was continued until the yeast could withstand temperatures significantly higher than the natural yeast from which they were derived.When these evolved yeast were immediately subjected to the original temperatures they had naturally belonged too, they died! They had lost their natural ability to withstand low temperatures, opting instead for genes which would provide protection against heat.
Similar to this classic experiment, I propose trying to force the evolution of yeast, or other rapidly multiplying organisms to change an important aspect of their biochemistry specifically. For example, in the case of ammonia as a solvent: Start by subjecting the yeast to an ever decreasing water supply and a gradually introduction of higher percentages of ammonia. Simultaneously the pressure in the system should increase to allow a significant portion of the ammonia to be liquid and usable as a solvent. My intuition tells me, that if this is done very slowly, and possibly through a specific sequence, an Earth life form will adapt and eventually take on a truly alien methodology.