Anomeric Effect
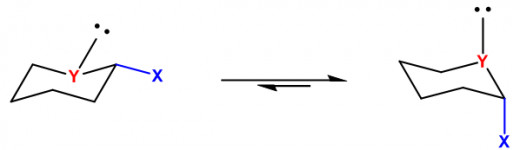
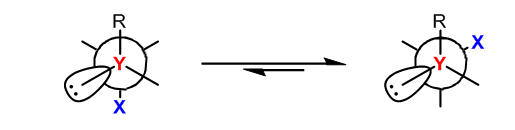
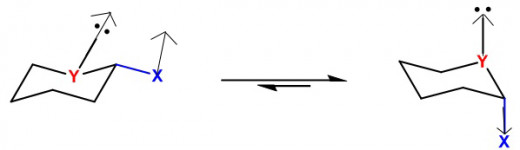
What is Anomeric Effect? It states that an axial anomer is more stable than the equatorial anomer. Well what is a anomer? An anomer is a cyclic stereoisomer, in many cases an alpha or beta stereoisomer of a pyranose form of a sugar. The reason why the axial position is preferred is simply because in an equatorial configuration there is a presence of dipole with any atom that isn’t a carbon or hydrogen, or a heteroatom. The dipoles of the heteroatom and the hydroxy group become parallel in such a way that it causes repulsion between atoms which is not good for the stability of the molecule. Now in axial position the dipoles points the other way, and as a result this is a good stabilizing effect for the molecule. The hydroxy group as a result adopts a gauche orientation. The carbon from which the hydroxy group becomes axial is known as the anomeric carbon. Now you might say, do not groups on a cyclohexane ring want to be in an equatorial position to relieve steric strain? This may be true, but when a heteroatom joins adjacent to a group like a hydroxyl group, the hydroxyl group becomes axial regardless of the steric strain, as stability of the entire molecule is important than mere strains. This is supported by numerous experiments done throughout the 50’s. The second reason why axial position is preferred has to do with delocalization of electrons. Delocalization provides stability because the electrons are able to move based on surrounding charges to better provide stability. When you have a heteroatom like oxygen with lone pairs in axial position, the electrons are able to move around with the electrons of the hydroxyl group that are also in axial position. This lowers the overall reactive energy of the molecule and provides great stability to the molecule. Now you might ask why doesn’t an every C-H group become axial if it provides better stability. Well it is because C-H groups aren’t good electron donors, heteroatoms are better donors because in many cases they’re rich in electrons and have high electronegativity. How the effect came to be discovered is quite interesting. John T. Edward and Raymond Lemieux in 1956 were studying carbohydrate chemistry, and they noticed how in acetyl group (COCH3) and in alkoxy group (R-O) groups like (CH3O-), tended to have a high percentage of axial positions. Upon studying and experimenting they came up with the postulate that it was the lone pairs from the oxygen that had played a role in the effect. Edward credits Lemieux with coming up with the name.
Bibliography:
1) Juaristi, E.; Cuevas, G. The Anomeric Effect, CRC Press: BOca Raton, 1995.
2) G.R.J. Thatcher (ed.), The Anomeric Effect and Related Stereolectronic Effects. ACS Symposium Series #539, 1993.
3) Juaristi, E., Cuevas, G., Tetrahedron, 1992, 48(24), 5019-5087.
4) Edward, John T. The Anomeric Effect and Associated Stereoelectronic Effects, Chapter 1: Anomeric Effect How It Came To Be Postulated, 1993, pp 1-5. ACS Symposium Series, Volume 539
Watch 6:25 min into the video to hear about Anomers
Did this help you?
Cite this page
MLA Format
Soman, Stanley. "Anomeric Effect." ExpertsColumn on HubPages. HubPages, 25 Dec. 2012. Web. 27 Dec. 2012(Replace with the date of your access). <http://expertscolumn.hubpages.com/hub/Anomeric-Effect>.
APA Format
Soman, S. (2012, December 25). Anomeric Effect. ExpertsColumn on HubPages. Retrieved December 27, 2012(Replace with the date of your access), from http://expertscolumn.hubpages.com/hub/Anomeric-Effect