Atoms
Atoms
Chemistry is about understanding the behavior of matter in terms of atoms. Matter is what makes up our universe. Matter is made up of atoms, which are too small to see.
What Is An Atom?
Subatomic Particles
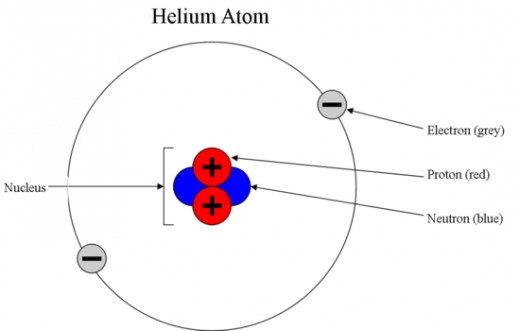
An atom is made up of even smaller particles – protons, neutrons, and electrons. Protons and neutrons are in the nucleus, a very tiny spot in the middle of an atom. Protons and neutrons are collectively called “nucleons”.
Electrons move very fast around the nucleus; it is convenient to visualize electrons as a cloud around the nucleus.
An atom is mostly empty space. The typical diameter of a nucleus is 10-14 – 10-13 m, while the diameter of the rest of the atom (the "electron cloud") is typically around 10-10 m. The diameter of each individual electron is estimated to be less than 10-18 m. To imagine the relative magnitudes of these numbers, imagine you are in a giant world where a typical atomic nucleus is 1 cm (or 10-2 m) instead of about 10-13 m. In this magnified world, the diameter of a typical electron cloud would be approximately 10 m (or 1000 cm) and each individual electron would be a tiny speck. Put another way, if you put two atoms side by side, the distance between the nuclei would be about 1000x larger than the nuclei themselves. Between the nuclei, where electrons are roaming about, is mostly empty space.
Example: A tennis ball has a diameter of 6.5 cm. If the nuclei of two neighboring atoms were the size of tennis balls, how far away from each other would the nuclei be?
Answer: About 1000x compared to the diameter: 1000x6.5 cm = 6.5x103 cm, or 65 m.
Elements
The word "property" refers to characteristics of matter, such as color, melting point, etc. In general, samples of matter that we encounter in nature can be separated into two or more components with different properties. However, there are samples that cannot be separated into two more more components with different properties. These samples are called elements. We explain why these samples cannot be separated into two or more distinct components by assuming that an element is made up of only one kind of atom.
There are a little over a hundred known elements to date; less than one hundred are naturally-occurring. Newly discovered elements are generally created in the laboratory and are not stable. Differences in properties of elements are due to differences in their atoms. All atoms of the same element have the same number of protons; atoms of different elements have different number of protons. An element's atomic number is defined as the number of protons in the each of the atoms that make up the element. Elements are listed in a periodic table in increasing atomic number (left-to-right, top-to- bottom). There are numerous periodic tables on the Internet. One site that provides an extensive compilation of data pertaining to the elements is webelements. As a matter of convenience, elements are assigned symbols that are derived from their names. The first letter of the symbol is capitalized and the second letter, if there is one, must be written in lowercase. For example, the symbol for oxygen is O. For magnesium, the symbol is Mg.
Example: What is the symbol for an element whose atoms have 10 protons in their nucleus?
Answer. If the atoms of an element has 10 protons in their nucleus, then this element has an atomic number of 10. Referring to a periodic table, we find that the element with atomic number of 10 is neon (Ne).
Example: How many protons are found in the nucleus of a magnesium (Mg) atom?
Answer: Referring to a periodic table, we find that the atomic number of magnesium is 12. Therefore, any atom of magnesium has 12 protons.
Isotopes
Atoms of an element all have the same number of protons. But they may have different number of neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes. To make a distinction between isotopes, we specify the total number of nucleons (protons and neutrons) next to the symbol for the element. This number, called the mass number. and is written either a superscript to the left of the symbol or following a dash after the symbol for the element.
Example: How many protons and neutrons are found in the nucleus of an atom of O-16 or 16O?
Answer: The number written is the mass number, which is the total number of protons and neutrons. Referring to a periodic table, we find that any oxygen atom has 8 protons. Therefore, everyatom of O-16 has 8 neutrons in its nucleus.
In general, naturally-occurring samples are not isotopically pure. For example, any sample of containing oxygen atoms will be found to have 99.757% O-16, 0.038% O-17, and 0.205% O-18. What this means is that out of every 100,000 O atoms, 99,757 would be of the O-16 variety, 38 of the atoms would be O-17, and 205 atoms would be O-18. These numbers are called the natural abundance of the oxygen isotope. A good place to look up this information is webelements.com. A modern instrument that is used to separate isotopes is a mass spectrometer. Here are some websites that describe the principles behind the operation of a mass spectrometer:
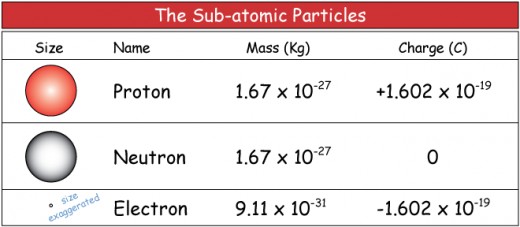
Masses of protons, neutrons, and electrons
Not only are electrons very small in size, they also contribute very little to the mass of an atom. The currently accepted masses of protons, neutrons, and electrons are given below.
- Mass of proton : 1.6726 x 10-27 kg, or 1.0073 u
- Mass of neutron: 1.6749 x 10-27 kg, or 1.0087 u
- Mass of electron: 0.00091x10-27 kg, or 0.0005486 u
The unit "u" is an abbreviation for "atomic mass unit," which is also frequently abbreviated as amu. You can see from the numbers given above that a proton’s mass is about 2000x that of an electron (1836x to be more precise). On the other hand, a neutron and a proton have almost the same mass. To the nearest tenth of an atomic mass unit, we can say that the mass of proton or neutron is 1.0 u, while that of an electron is 0.0 u. This is why the number of electrons is ignored in the defining the mass number of an atom. To the nearest atomic mass unit, the mass of an atom is equal to its mass number.
Example: which has a largest total mass: 1 proton, 3 electrons, or 2 neutrons?
Answer: The mass of one proton is 1.0 amu. Three electrons have a total mass of 0.0 u. Two neutrons have a total mass of 2.0 u. Of the choices given, 2 neutrons have the largest total mass.
We cannot get the mass of an atom by simply adding the masses of protons, neutrons, and electrons that make it up However, to the nearest atomic mass unit, it is approximately equal to the mass number. The actual mass of an atom is slightly different and needs to be looked up. The discrepancy is called the "mass defect" (m) and is related to the "binding energy" (E, the energy needed to separate all protons and neutrons from one another) by Einstein's equation: E = mc2. In this equation c is the speed of light, 2.998x108 m/s.
Example: what is the mass of an atom of 18O?
Answer: to the nearest atomic mass unit, the mass of an 18O atom is 18 u; if we were to look it up, we find that it is equal to 17.9991603 u. Note that the sum of the individual masses of 8 protons and 10 neutrons alone should be larger than 18 u, but an actual O-18 atom has a mass slightly less than 18 u.
Isotopic Masses
Most elements have two or more naturally-occurring isotopes. The average mass of atoms in a naturally-occurring sample of an element is typically listed in simple periodic tables. The numerical value of the average mass (in u or amu) is called the atomic weight. For example, the atomic weight of Mg is often listed as 24.305. This means that:
In a naturally-occurring sample of Mg, the average mass of the atoms is 24.305 u.
There is not a single Mg atom that has a mass of 24.305 u.
If we want information about specific isotopes of an element, we need to look it up. For example, we can look up the following information about naturally-occurring isotopes of Mg from webelements.com:
- Mg-24, mass=23.99 u, relative abundance = 78.99%
- Mg-25, mass=24.99 u, relative abundance = 10.00%
- Mg-26, mass=25.98 u, relative abundance = 11.01%
A relative abundance of 78.99% for Mg-24 means that about 79 out of every 100 atoms have a mass of of 23.99 u. The average mass of can be calculated from the information above using the formula:
- Average Mass = Sum of (relative abundance x mass)
In other words,
- Average Mass of Mg = (78.99% x 23.99) + (10.00% x 24.99 u) + (11.01% x 25.98%) = 24.31 u
Radioactivity
Neutrons are needed to stabilize a nucleus. If there are too many or too few neutrons, the atom would be unstable. The nuclei of unstable atoms tend to break up (or undergo nuclear fission) over time. These atoms are called radioactive. A good source of information regarding radioisotopes is webelements.com.
It is tempting to think that there would be no naturally-occurring radioisotopes since they are unstable. In fact, there are naturally-ocurring radioisotopes. These are the ones that have extremely long half-lives. The half-life of a radioactive atom is the time it takes for half of of the atoms to decay (transform into something else). For example, U-238 has a half-life of 4.46 billion years. This means that half of U-238 that existed 4.46 billion years ago are still here. Less stable radioisotopes can be created in the laboratory by bombarding atoms with high energy particles (neutrons from a nuclear reactor, or charged particles accelerated within a cyclotron). For more information, Click here
Electrical Charge
Electrons and protons are said to be electrically-charged, while neutrons are electrically-neutral. The unit for electric charge is Coulombs (official abbreviation: C). Electrons carry the smallest magnitude of charge known, which is –1.6x10-19 C. This amount of charge is usually assigned a value of “-1” (atomic units). Protons have a charge of +1.6x10-19 C, or “+1” (atomic units). Neutrons are not charged (charge=0). All atoms are electrically neutral. This means that they have equal numbers of protons and electrons.
Example: An atom has 10 protons. How many electrons does it have?
Answer: An atom is electrically neutral. It has equal numbers of protons and electrons. If an atom has 10 protons, it also has 10 electrons.
Example: What is the nuclear charge of a magnesium atom?
Answer: Referring a periodic table, we find that the atomic number of magnesium is 12. Therefore, any magnesium atom has 12 protons in its nucleus. Since each proton carries a charge of +1, the charge of the nucleus is +12.
© 2015 Discover the World