Atoms, Elements, Compounds and Molecules
Intro
Everything in the universe is made out of atoms. From living things to non-living things. Atoms react with one another to form elements, compounds and molecules. Knowledge of atoms can help us predict the outcome of a chemical process.
Atoms
An atom is a small particle that is used to make the entire universe. Recently, It was discovered that an atom is made out of even smaller particles. These particles are protons, neutrons, electrons. The protons carries a positive charge while an electron carries a negative. Neutron carry neutral charge. The charge of a normal atom always stays neutral. Each atom will have a different number of protons, neutrons and electrons. Look up the periodic table for this. The number on the top left corner is the number proton. Because a normal atom will always have neutral charge, the number of electrons will always be the same as the number of protons. If oxygen has 8 protons, that means it also has 8 electrons. To know the the number of neutrons minus the number of protons with atomic mass (which can be found under the symbol). If oxygen has an atomic mass of 16 and 8 protons. (16 - 8 = 8) 8 would be the answer.
Elements
Elements are two atoms of the same type chemically joined together. Examples are H2 and O2. There are currently 118 different elements. Remember elements are made out of only one type of atom.
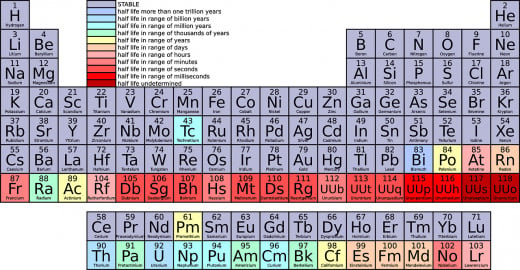
The Periodic Table of Elements
Compounds
Compounds are substances that are made out two or more of different atoms.An example of a compound is H2O. There are two hydrogen atoms and one oxygen atom in this compound. This compound is a covalent bond. We will discuss this in another hub.
Molecules
Molecules are two or more atoms chemically joined together. Molecules unlike compounds can also be made from atoms of the same type. Elements and compounds are also considered molecules as they have more than two atoms.
Summary
- Atom are the building blocks of the universe.
- An atom has three types of sub-atoms. Protons, neutrons and electrons.
- An element has two of the same atoms.
- An compound has two or more of the different atoms.
- An molecules has two or more of any atoms.
Thank you for reading and have fun studying.