Atoms and Molecules
Atoms
Like I menioned in my other post, anything that you can see with the naked eye or even the best microscope is a bundle of atoms too many to ever count. You can make an accurate guess as to how many there are - but that's still guessing. I likened it to counting the grains of sand on the beach - there are just too many to count.
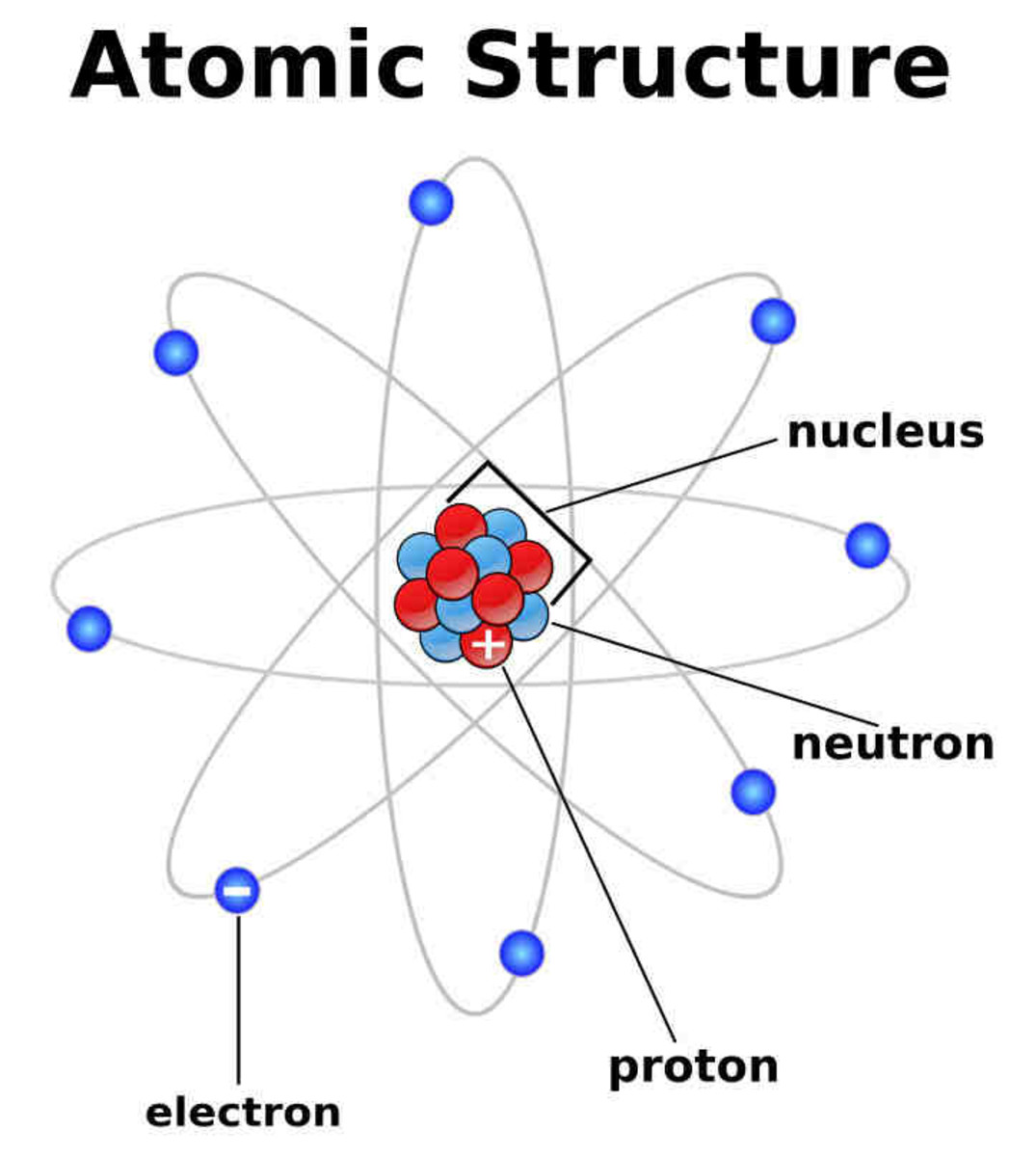
But what do atoms and molecules really look like? What are they made of and how do they work? There are two main parts that chemists look at - three if you're a nuclear chemist or paying attention to isotopes. In an atom there is the nucleus and the electrons that orbit it. People have this image that it's like the solar system where the sun is the nucleus and the planets are the electrons. This is not entirely inaccurate, but it's not a good idea to embed this image in your mind.
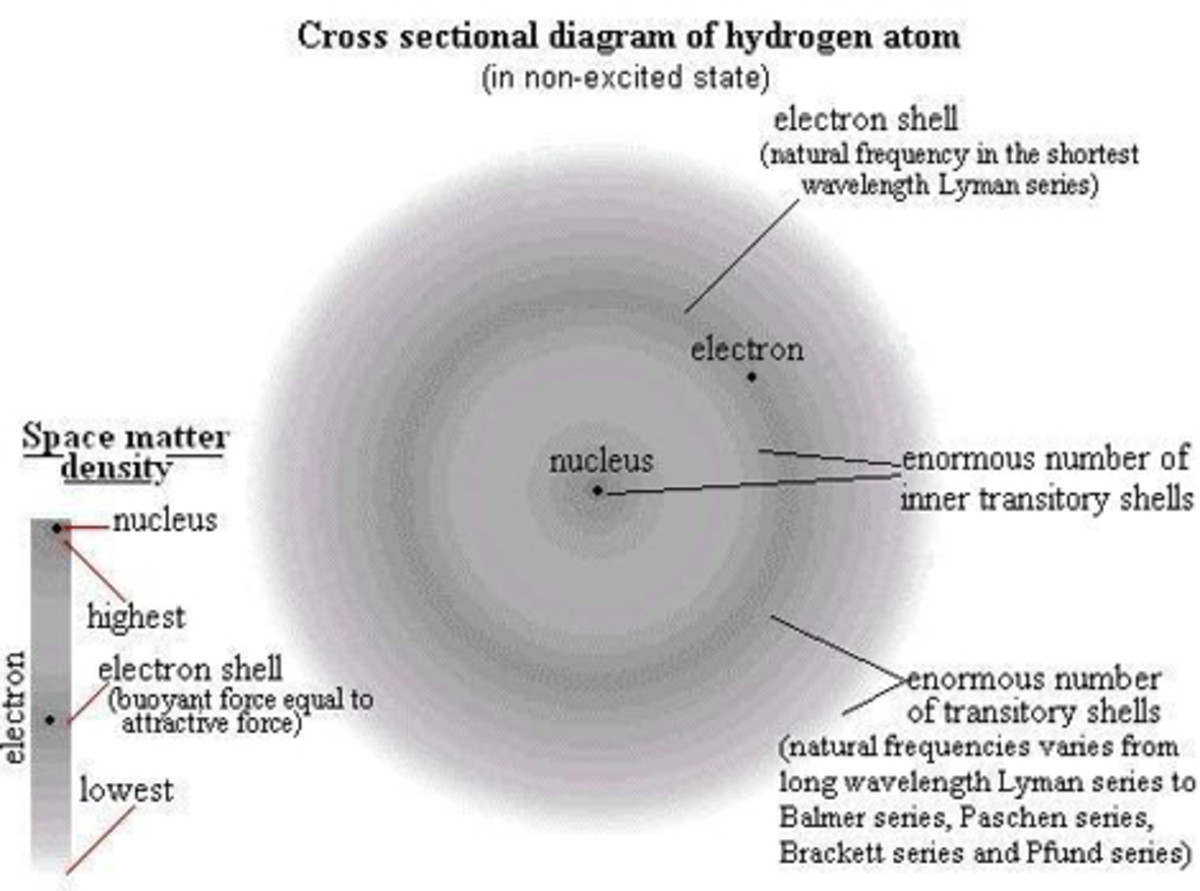
We do not know exactly what an atom looks like, but we have a highly educated guess that is built off of large-scale models. Electrons orbit the nucleus (and there's a debate whether the word "orbit" is appropriate) in a harmonic way. So by studying sound and waves, scientists were able to derive what an electron should look like as it moves around the nucleus. Schrodinger even came up with an equation to map it out. See the image above of a simple electron cloud.
Protons and Electrons
Protons and electrons are the charged subatomic particles that define how an element reacts. Protons are the positively charged particles that are contained in the nucleus of an atom. Electrons are negatively charged and are far outside the nucleus. The size of the nucleus is incredibly small compared to the electron cloud around it. I once heard it likened to a pea in a football stadium.
Which element you're talking about is completely dependent on how many protons are in the nucleus. Hydrogen is the first element and atoms of this element have only one proton in the nucleus. Helium atoms have two protons in the nucleus. Lithium has three. Beryllium has four. So on and so on. If you look at a periodic table of elements, reading it left to right and top to bottom, you will see all the elements numbered off by how many protons they have in their nucleus.
A lot of people think of the four elements of wind, water, fire, earth. These are not elements to a chemist! No Bruce Willis shit or a fifth element here. Elements can be thought of as types of atoms, where the number of protons determines what element you're talking about.
Neutrons are heavy particles in the nucleus that hold no charge and don't play a significant role in simple chemistry. They do, however, mean that the weight of a nucleus will be greater than the number of protons contained in the nucleus. Long story. Irrelevant for now. Just thought I'd mention them.
So protons are heavy and positively charged. Electrons are tiny and negatively charged. When two atoms interact, it's mostly the electrons that do the interacting. The electrons are all around the atom and moving so fast that pinpointing a location is a bit impractical. Instead, we look at all the places it could be and map it out - and all those places combined look sort of like a cloud. That's why we refer to it as the "electron cloud". When two atoms get close enough to interact, the electron clouds of each are the first parts to touch each other.
Now a bit about energy again. Energy is god when it comes to particle interactions. Things tend to give off energy - objects fall, fast things slow down, sound gets quieter the farther from the source it gets... If it can move to lower energy levels, it will. If two atoms can lower their energy by sharing their electrons, they will share their electrons and kind of attach themselves to each other while doing so. This is refered to as bonding.

Molecules
When two or more atoms bond, the result is a molecule! It's as simple as that, but not really. There are multiple ways atoms can bond together. One way is for them to share electrons evenly between the two - a perfect balance only happens with atoms of the same element. Another way is for them to share electrons unevenly - one atom holds the electrons more tightly than the other. Yet another way is for one atom to rip off an electron completely from the other atom, causing the stronger atom to have one more electron than proton and be negatively charged while the weaker has one less electron than proton and be positively charged - since negative and positive attract, the two charged atoms will stick around each other. And believe it or not, metals even have their own type of bond that is very similar to the first described.
Listing them off respectively as I described: Nonpolar covalent bond, polar covalent bond, ionic bond, and metallic bond.
Two oxygen atoms bound together (O2) share their electrons equally and have a nonpolar covalent bond. Two hydrogens bound to an oxygen (H2O - water) share electrons unevenly and have a polar covalent bond. A sodium attracted to a chloride (NaCl - table salt) literally has the electron ripped off and they have an ionic bond.
Metals are unique because an electron in the outer parts of the atoms is shared by all the atoms in that metal, which can be trillions upon trillions of atoms. The electrons are not assigned to any one atom and can flow around a bit like a fluid. That is why metals make the best conductors of electricity. That is what electricity is - a massive flow of electrons from one area to another. Pretty cool, huh?