Biology - Protein Structure
Protein Structure
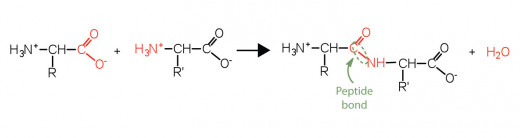
To make proteins lots of amino acids bond together in a specific sequence that is determined by the DNA.
The amino acid chains get longer and longer as more amino acids bond to it.
Parts of the amino acid chain will start to coil and pleat, the amount of coiling/pleating depends on the types of amino acids in the chain - the primary structure.
The coils and pleats are held together by numerous hydrogen bonds give the chain stability.
Once the whole chain is complete and a polypeptide is formed the coils and pleats will come together and give the molecule a 3D structure which is again determined by the primary structure of the protein and is held together by a number of different bonds.
There are 4 different levels of protein structure:
- Primary
- Secondary
- Tertiary
- Quaternary

Primary and Secondary Structure
- The primary structure of a protein is the specific sequence of the amino acids that form it.
- The secondary structure is when the chain of amino acids either forms an 'alpha helix' or a 'beta pleated sheet'. The secondary structure is defined by the patterns of hydrogen bonds between backbone amino and carboxyl groups. Hydrogen bonds, despite the fact that they're weak, hold the coils in place because there are so many of them overall it gives great stability.
Tertiary Structure
- When the coils and pleats themselves coil or fold, it creates a 3D shape.
- This shape is held together by many different bonds.
- The tertiary structure is vital to it's function - for instance a hormone must be a specific shape to fit with the hormone receptor on the target cell.
- Structural proteins such as collagen has proteins chains that are wound around each other, this provides strength.
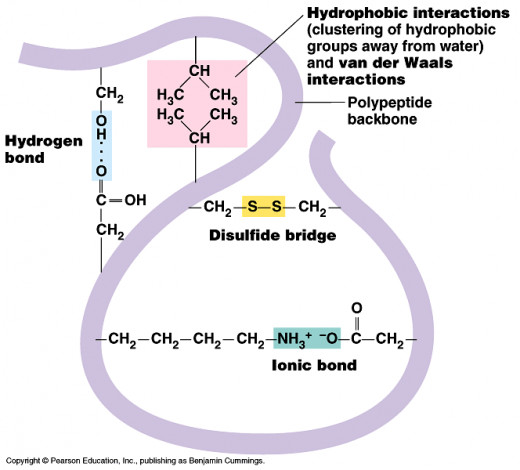
Bonds Within Tertiary Structure
Disulfide Bonds:
- The amino acid cysteine contains sulfur.
- When two of cysteine molecules are close together disulfide bonds can form.
Ionic Bonds:
- R-groups on the amino acids can sometimes carry a charge.
- When oppositely charged amino acids are found close to each other, ionic bonds can form.
Hydrogen Bonds:
- When positively charged groups are found next to negatively charged groups, hydrogen bonds form.

Different Protein Structures
The three-dimensional shape of a protein will either be in the 'globular' category or the 'fibrous' category.
Globular proteins tend to roll up into a compact ball like structures where any hydrophobic R-groups will turn inwards whilst the hydrophillic ones with turn outwards.
This makes it easier for the water molecules to cluster around them, thus making them water soluble molecules.
Globular proteins usually have metabolic roles and are found in plazma proteins, antibodies and in the blood of mammals (haemoglobin).
Fibrous proteins form fibres most of which have repetitive sequences of amino acids.
Fibrous proteins are insoluble in water and usually have structural roles and are found in things like collagen, bone and cartilage.
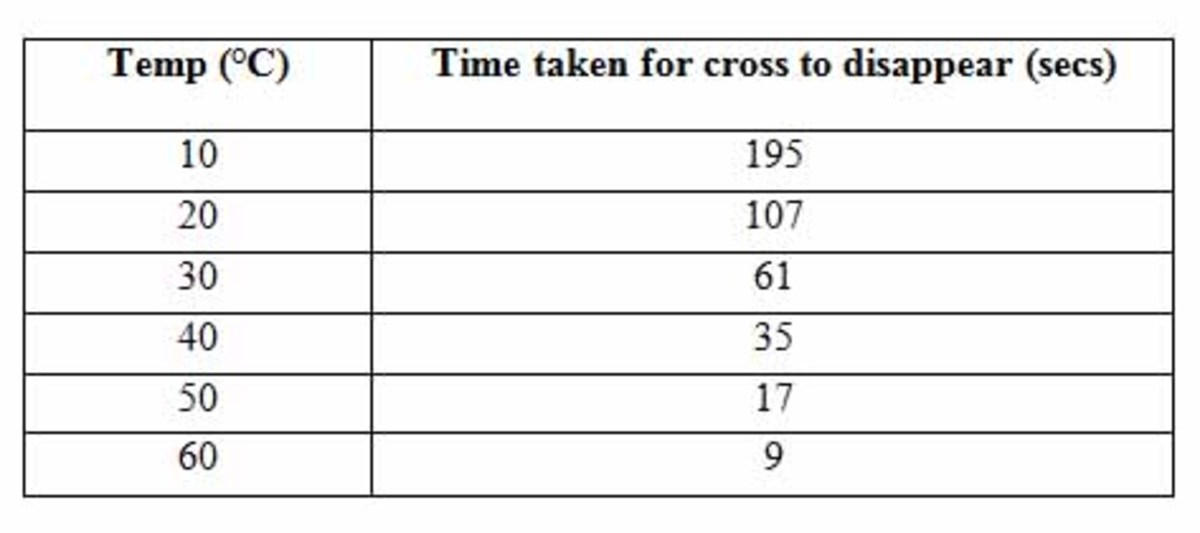
Proteins and Heat
When a protein is heated it is given kinetic energy and vibrates.
This causes some of the bonds within the tertiary structure of the molecule to break.
The bonds within the structure are not covalent and are therefore very weak and not much heat is needed to break them.
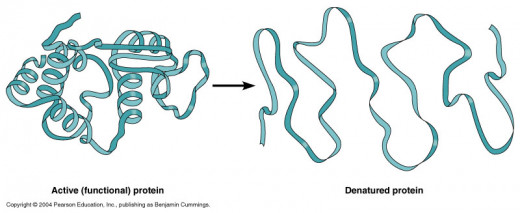
If enough heat is applied to the protein, the whole tertiary structure will unravel and uncoil and it will no longer be able to function - this is called denaturation.
If it is cooled the protein will not be able to reassemble itself.