Chemical Concept of Electromeric Effect
List of Topics Included
(1) What is called electromeric effect?
(1/A) Understanding the terms, “substrate” and “reagent”
(2) Various types of electromeric effects and their symbols
(2/A) Pictorial representation of +E and -E effects
(3) Some characteristics of electromeric effect
(4) Understanding the meaning of electrophile and nucleophile
(5) Illustration of +E effect
(5/A) Equations showing mechanism of reaction between ethene and chlorine
(5/B) Evidence for existence of carbocation
(5/C) Pictorial representation of equations supporting existence of carbocation and +E effect
(6) Illustration of -E effect
(7) Difference between inductive effect and electromeric effect
(7/A) Table showing main points of difference between inductive effect and electromeric effect
(8) References
(1) What is Called, “Electromeric Effect?”
Definition of electromeric effect:
“The complete and spontaneous transfer of shared pair of π-electrons of a multiple bond, to one of the bonding atom, at the time of approaching reagent, is called electromeric effect”.
It is also known as E-effect. It is depicted by a curved arrow.
Due to complete transfer or migration of two π-electrons on one of the bonded atom, this effect results in development of unit positive and unit negative charges on bonded atoms.
This results in increased attraction between substrate and reagent which in turn, helps chemical reaction.
This means, electromeric effect, always facilitates a chemical reaction. In other words, it increases chemical reactivity of substrate.
(1/A) Understanding the terms: “Substrate” and “Reagent”
Whenever a chemical reaction takes place, there are two participating species:
(1) Substrate and
(2) Reagent
Out of these two species, the one which is under observation, is called, “substrate”. In other words, substrate is part of our study because we are very much interested to know what happens to this particular species after reaction is over.
A species under investigation, is called, “substrate”.
On the other hand, reagent is a tool through which changes are introduced to substrate so as to gain knowledge about some of its chemical characteristics.
This will be clear from following example.
Consider a reaction between benzene and chlorine in presence of catalyst, "ferric chloride". As per following equation, the products of this reaction are chlorobenzene and hydrogen chloride.
C6H6 + Cl2 = C6H5Cl + HCl
In this reaction, benzene is under study hence it is called a substrate. We carry out this reaction to know what happens to benzene when treated with chlorine.
However, we do not bother, what happens to chlorine when it reacts with benzene. This means chlorine is not under investigation. Hence, chlorine is not a substrate but is a reagent.
(2) Various types of Electromeric Effects and their Symbols
There are two types of electromeric effects as described below.
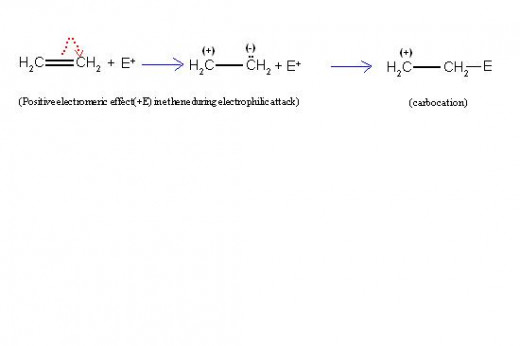
(1) Detailed information about +E effect:
If the attacking reagent is an electrophile, it will attract the electron pair of π-bond due to its positive charge or due to its electron deficiency.
In this case, there will be electrostatic attraction between electrophile and electrons of π-bond. Hence the transfer of two electrons of π-bond of substrate molecule will take place on such an atom of a multiple bond, which is nearer to attacking reagent.
This will cause one unit negative charge on atom which gains electron and also simultaneously one unit positive charge on another atom of π-bond.
This is called, "positive electromeric effect" and is represented by symbol, “+E effect”.
It always produces carbocation intermediate.
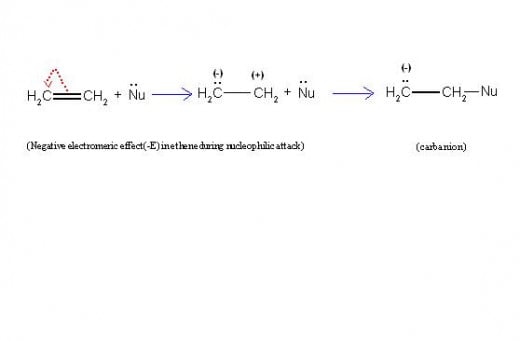
(2) Detailed information about -E effect:
If the attacking reagent is a nucleophile, it will repel the electron pair of π-bond due to its negative charge or due to its high electron density. In this case, the transfer of two electrons of π-bond of substrate molecule will take place on such an atom of a multiple bond, which is away from the attacking reagent.
This will cause one unit positive charge on atom which looses electron and also simultaneously one unit negative charge on another atom.
This is called, "negative electromeric effect" and is represented by symbol, “-E effect”.
It always produces carbanion intermediate.
This will be clear from following pictures.
Pictorial representation of +E Effect
Pictorial Representation of -E Effect
(3) Some Characteristics of Electromeric Effect
(1) The transfer of electrons taking place during electromeric effect is purely temporary. It occurs only at the time when attacking reagent comes closer to a multiple bond.
This means, if the attacking reagent is removed, both of the π-electrons will occupy their original position to form a multiple bond again.
[Note: Being temporary, electromeric effect does not affect any of the physical properties of substrate molecule.]
(2) This effect always favours the phenomenon of bond formation, thus it helps chemical reaction to complete.
(3) It is observed only during an addition reaction of a substrate having multiple bond containing π-electrons (for example: carbon-carbon double bond, carbon-carbon triple bond, carbon-oxygen double bond etc).
(4) The reagent being added to multiple bond (which is also called, addendum) must be polar or an ionic reagent carrying either (a) positive charge (known as an electrophile) or (b) negative charge (known as nucleophile).
(4) Understanding the Meaning of, "Electrophile" and "Nucleophile"
In the study of organic chemistry, the reagents are commonly classified into two major categories as explained below:
(1) Electrophiles: The meaning of electrophile is, “electron loving”.
They have strong attraction for negative charge. These are the species either bearing a positive charge on them or are neutral molecules which are electron deficient.
Due to having positive charge or electron deficiency, they always try to combine with some negatively charged or electron rich species to become stable.
They are also known as, “Lewis acid”.
Examples of electrophiles are: H+, Cl+, Na+, NO2+, AlCl3, FeCl3 etc.
(2) Nucleophiles: The meaning of nucleophile is, “positive charge loving”.
They have strong attraction for positive charge. These are the species either bearing a negative charge on them or are neutral molecules which have excess electrons.
Due to having negative charge or having excess electrons, they always try to combine with some positively charged or electron deficient species to become stable.
They are also known as, “Lewis base”.
Examples of nucleophiles are: OH-, Cl-, CN-, NH3, H2O etc.
(5) Illustration of +E effect
To understand +E effect properly let us consider the electrophilic addition reaction taking place between ethene with bromine.
If this reaction involves +E effect, then the intermediate formed must be a carbocation.
In other words, presence of carbocation as an intermediate during this reaction is evidence of +E effect.
Here it is possible to show experimentally that carbocation exists during this reaction.
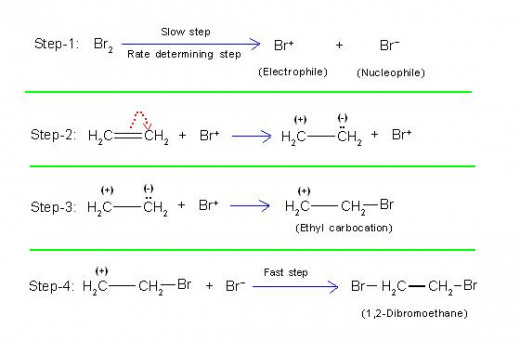
This is proved by the mechanism of this reaction as described below.
Step-1: Bromine is first divided into Br+ (electrophile) and Br- (nucleophile).
[Considering the case in detail, the positive end of polarized bromine combines first with ethene, followed by departure of Br- nucleophile].
Step-2: Then electrophile (Br+) approaches ethene, causing +E effect in ethene.
Step-3: Br+ now combines with negatively charged carbon to form “ethyl carbocation” intermediate.
Step-4: Ethyl carbocation finally combines with Br- nucleophile to give product,
“1,2-dibromoethane”.
The pictorial representation given below will make this clear.
(5/A) Equations Showing Mechanism of Reaction Between Ethene and Chlorine
(5/B) Evidence for Existence of Carbocation
Existence of carbocation during this reaction is actually an evidence of +E effect.
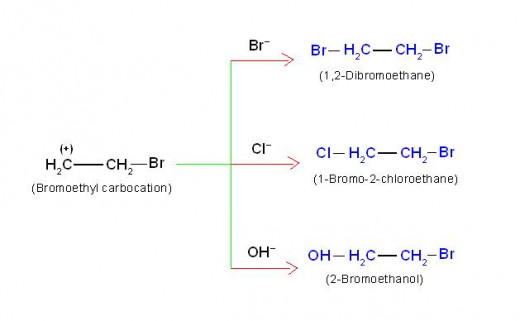
In the reaction illustrated above, if bromoethyl carbocation really exists as an intermediate, it can react not only with bromide nucleophile, but can also react with other nucleophiles if present in the reaction mixture. This may give extra products corresponding to other nucleophiles.
In order to confirm this, two other nucleophiles: Cl- and OH- were deliberately added in the reaction mixture in the form of NaCl and H2O.
Here NaCl will provide Cl- nucleophile while H2O will provide OH-nucleophile.
As per expectation, two additional products: (a) 1-bromo-2-chloroethane and (b) 2-bromoethanol were also formed along with 1,2-dibromoethane.
This experimental observation strongly supports existence of carbocation and +E effect in ethene.
This will be clear from following equations.
(5/C) Equations of chemical reactions which support +E effect
(6) Illustration of -E effect
The nucleophilic addition reaction between ethanal (which is also known as acetaldehyde) and hydrogen cyanide to yield “acetaldehyde cyanohydrin” is an example of -E effect.
Here, oxygen of carbon-oxygen double bond becomes negatively charged due to -E effect caused by cyanide nucleophile.
CH3-CH=O + HCN → CH3-C(H)(OH)-CN.
The other aspects of this reaction can be shown as shown above in case of +E effect.
(7) Difference Between Inductive Effect and Electromeric Effect.
In case both inductive as well as electromeric effects are operating simultaneously in the same molecule, but they are in the opposite directions, then it is the electromeric effect which will dominate inductive effect.
The reason is, a complete transfer of shared electrons occurs in electromeric effect, while a slight displacement of shared electrons occurs in inductive effect.
The following table shows main points of difference between these two effects.
(7/A) Table showing main points of difference between Inductive and Electromeric effects
Serial number
| Inductive effect
| Electromeric effect
|
|---|---|---|
1
| It is observed in compounds containing single bond
| It is observed in compounds containing double or triple bond
|
2
| It is permanent effect
| It is temporary effect
|
3
| It takes place due to presence of electron withdrawing or electron donating group attached to the end of carbon chain
| It takes place due to positive or negative charge of attacking reagent
|
4
| It causes slight displacement of electrons bonded through sigma-bond
| It causes complete transfer of electrons bonded through pi-bond
|
5
| The effect of charge developed extends upto forth carbon atom of the chain
| The effect of charge developed remains limited on the same carbon atoms
|
6
| It affects physical properties of substance
| It does not affect physical properties of substance
|
Can You Answer?
view quiz statistics(8) References
(1) Organic Chemistry by Robert Thornton Morrison and Robert Neilson Boyd, Seventh edition, published by Pearson Education, Inc.
(2) I. I. T. Chemistry, by Dr. O. P. Agarwal and Avinash Agarwal, 135th Edition, Jai Prakash Nath Publication, Meerut, India
(3) Pradeep's New Course Chemistry, Twenty Sixth Edition, Class- XI, Volume II, by S.N. Dhawan, S.C. Kheterpal and P.N. Kapil, Pradeep Publication, Jalandhar, India
(4) Fundamentals Of Chemistry, Class 11, by J. D. Lee, Solomons & Fryhle, Published by: Wiley India Pvt. Ltd., 4435-35/7, Ansari Road, Daryaganj, New Delhi-110002
(5) Modern's abc of Chemistry, For Class XI, Part-II, by Dr. S. P. Jauhar, Published by: Modern Publishers, MBD House, Railway Road, Jalandhar City, India
(6) Nootan ISC Chemistry, Class XI, by Dr. H. C. Srivastava, Published by: Nageen Prakashan (Pvt.) Ltd., 310, Western Kutchery Road, Meerut-250001, U.P., India
Next:
- Lucid Chemistry of Tautomerism
Tautomerism is a special kind of isomerism in which both the isomers (called tautomers) co-exist at any given time and also remain in equilibrium with each other.
Previous:
- Complete Chemistry of Inductive Effect Observed in Organic Molecule.
After studying this hub, one can easily answer the questions like: (a) why methanoic acid is stronger acid than ethanoic acid? (b) why tertiary carbocation is more stable than primary carbocation?