Intro to College Chemistry

Intro to College Chemistry
I am studying for my chemistry final and I thought making a lens about Intro to chemistry would help me remember and study the information and help others learn about it too.
In this lens I will talk about properties of matter, 3 origins of chemistry, Basics of Energy, as well as some other information.
Hope this helps.
Thank you very much for your support!!!
Properties of Matter
Matter is all the "stuff" that makes up the universe
*All matter is composed of some combination of elements.
Elements are something made purely of one substance.
These are shown on the periodic table.
Physicial Properties: The characteristics that a substance shows without an outside substance interacting with it.
Physical Change: An alter of its physical form but not its composition.
(ex. ice > water, water > water vapor)
Chemical Properties: The characteristics of a substance when interacting with other substances.
Chemical Reaction: When the original substance is converted into another substance through chemical interaction.
The Three States of Matter
Gas:A substance that conforms to the shape of a container but also fills the whole container.(It doesn't form a surface)
Liquid:A substance that conforms to the shape of a container but does not occupy more than its volume.
Solid: A substance with a fixed shape that does not conform to the shape of a container around it.
Basic Principles of Energy
Potential Energy: The energy an object has due to its position. (ex. water behind a damn has high potential energy.)
Kinetic Energy:The energy an object has due to movement (ex. A waterfall has a high kinetic energy when gravity acts on the water to fall.)
Look at the section on Thermochemistry for more calculations with Energy!!
3 Origins of Chemistry
1) Alchemy:
What it is: Started in the 1st Century AD by the Greeks. Alchemists believed that materials found in nature naturally strive to become more perfect.
Benefits:
---Invention of devices used to distillate, percolate, and extract still used today.
---They also encouraged observation and experimentation.
2) Medicine:
What is it: In the 13th Century extracted roots, herbs and other plants were used medicinally. Later they introduced minerals drugs for treatments
Benefits:
---Created alliance between medicine and chemistry that exists today.
3) Technology:
What is it: Pottery, dyeing and Metallurgy contributed to understanding the properties of materials. They used introduced quantitative measurements to describe the materials and process for making the materials.
Benefits:
---Started asking questions about material properties.
---Started writing and calculating based on material properties.
The Scientific Method
What is the Scientific Method?
This is an guideline for conducting and testing a hypothesis in a objective, and verifiable way.
Step 1 Observation:
Observations are data collected through different means for use in a hypothesis testing. Often there is quantitative data that can be compared.
Step 2 Determine your Hypothesis:
Based on your observation you can now come up with an idea of what is happening or what will happen in an experiment.
Step 3 Experiment:
A clear set of steps that are used to test the hypothesis with experimentation. The hypothesis can be revised but the results of the experiment can not be changed.
Step 4 Model:
The formation of model or theory based on the findings of the experiment. These can be used to predict other experiments down the road and may be tested to calculate a need to revise the hypothesis. Even if something has been proven many many times it can always have a chance of being disproven through the scientific method.
SI Units
These are the standard (SI Units) agreed upon units for each type of measurement in the scientific community.
measurement type ----------Unit name + Abbreviation
Mass-------------------------------- kilograms (kg)
Length------------------------------ meter (m)
Time-------------------------------- second (s)
Temp-------------------------------- kelvin (K)
E. Current--------------------------- ampere (A)
Amount of substance--------------- mole (mol)
Luminous Intensity----------------- candela (cd)
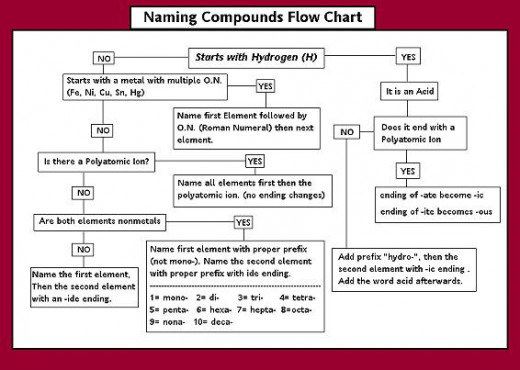
How to name compounds
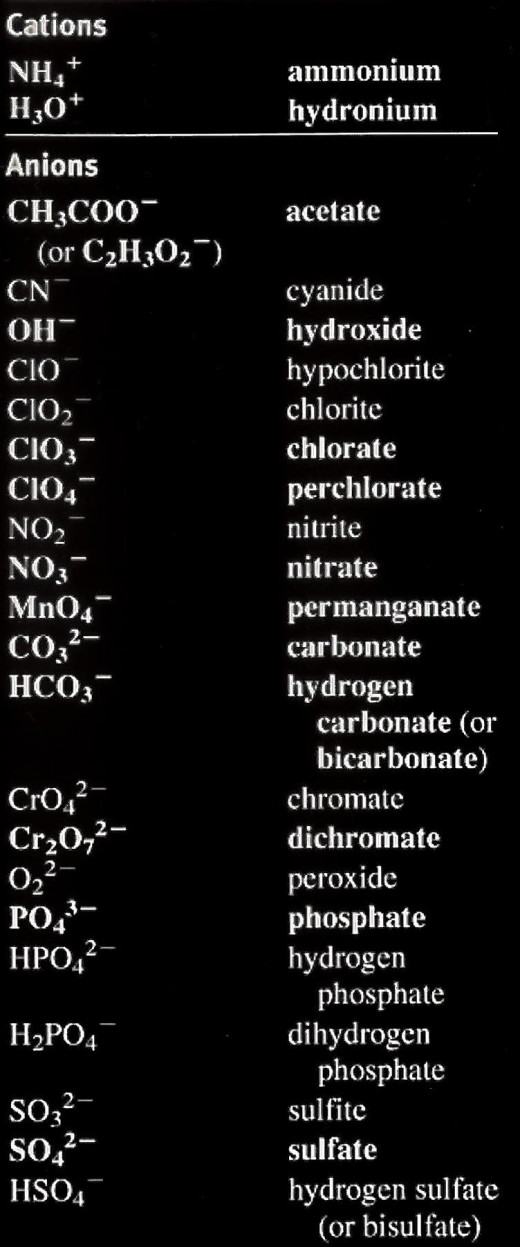
Important Cations and Anions
Cations = positive charge & Anions = negative charge
Thermochemistry
System: This is what we are interested in studying (ex. Chemicals, mixture, solution, etc...)
Surrounding: This is everything that is not a part of the system.
(ex. The room, a person, the beaker, tools used for measurement, etc...)
Energy = E
Change in E = Final Energy - Initial Energy
Change in H = Final Heat - Initial Heat
If the change in E < 0, then Final Energy < Initial Energy
If the change in E > 0, then Final Energy > Initial Energy
Both Heat and Work are a type of energy transfer
Heat = q
Work = w
Change in E = q + w
Acids and Bases
An acid/base reaction is one in which both the acid and base react to form water (H2O) and a salt (formed from the additional elements in the acid and the base)
Acids create a H+ ion when dissolved in water
Bases produce a OH- ion when dissolved in water
Strong Acids (fully dissociate in water)
-Hydrochloric acid (HCL)
-Hydrobromic acid (HBr)
-Hydroiodic acid (HI)
-Nitric acid (HNO3)
-Sulfuric acid (H2SO4)
-Perchloric acid (HClO4)
Strong Bases (fully dissociate in water)
-Sodium Hydroxide (NaOH)
-Potassium Hydroxide (KOH)
-Calcium Hydroxide [Ca(OH)2]
-Strontium Hydroxide [Sr(OH)2]
-Barium Hydroxide [Ba(OH)2]
Chemistry for Dummies books
Formulas
--Density= Mass/Volume
--Temp Conversion:
Celsius to Fahrenheit
(Temp in C)= 5/9((Temp in F)- 32)
Fahrenheit to Celsius
(Temp in F)= 9/5(Temp in C) + 32
--How to find # of Neutrons in an Atom
N= A -Z
n: # of neutrons
A: Mass #
Z: Atomic #
--How to find Molar Mass
MM =∑(atomic Masses)
MM: Molar Mass
∑: Sum
Chemistry Study Links
- Chemistry Study Cards
This site has useful study cards over many topics of Chemistry. - Chemistry Homework Help
Lots of help over different topics in Chemistry. - Wikipedia: Chemistry
The Topic of Chemistry on Wikipedia.