Development of Periodic Table
Introduction
As more elements began to get discovered a need arose to classify them in a way that their properties and chemical as well as physical behaviors get classified and studied. A lot of chemists tried to classify the elements on the basis of density, durability, malleability or metallic properties. However these classifications were not satisfactory because at times the number of elements falling in one particular group was too big. This did not serve any useful purpose. William Prout discovered in 1815 that the atomic weight of an element never changed and so using this atomic weight criteria could form a basis of classification. In 1829, J. W. Doberenier noticed that certain elements had a relationship between atomic weights and their properties and he grouped them in the combination of three called triads. In a triad the atomic weight was approximately the arithmetic mean of the atomic weights of the other two. For example calcium(40), strontium(88) and barium (137) mean:- ({40+137}/2) =88 (approx.). However the above classification failed as majority of elements could not form similar triads and even when they did, the properties of the elements had little relation.

Newlands’s Law of Octaves
In 1864, John Newlands arranged elements in ascending order of atomic weights. He observed that the eighth element has similar properties o the first element. This classification was called Newland’s Octaves. The advantage of the system was that properties of elements were related to their atomic mass an a periodicity was shown in the properties of the elements. The theory failed after the discovery of noble gases as now ninth element had similar properties. Also, Law of Octaves failed for heavier elements beyond calcium. In 1869, a Russian chemist Mendeleev arranged all the known elements in their increasing order of their atomic weight in a Periodic table. He left blanks in the periodic table where the properties of the elements did not match. He observed a periodicity of elements of similar properties a regular intervals. The law states that if elements were placed in increasing order of their atomic weights there is a recurrence of similar properties after every seventh element. In the Mendeleev's periodic table there were eight vertical columns called groups from Group I to Group VIII. All elements in a group has similar properties. The horizontal row was called a period. Mendeleev's periodic table had few gaps where properties were predicted by neighboring elements. The periodic table had few defects like anomalous pairs, position of isotopes, separation of chemically similar elements and ambiguous position of Hydrogen.
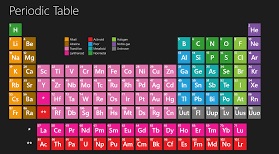
Modern Periodic table
Modern periodic law states that the physical and chemical properties of the elements are a periodic function of their atomic numbers. The law is based on the fact that when elements are arranged in ascending order of atomic numbers, elements with similar properties are repeated at regular intervals. Moseley discovered in 1912 that the frequency of X-rays emitted by a metal, when bombarded with high speed electrons, the square root of the frequency was proportional to the element's atomic number. The above observation established that atomic number was a better fundamental property of an element. Hence all modern periodic tables are based on atomic numbers rather than atomic weights. The long form of the periodic table is most accepted and used table for element classification. The periodic table has eighteen vertical columns known as groups. There are seven horizontal rows called periods. The table is a sub-group table for rare-earth elements called lanthanides and actinides. The merits of the long form of the periodic table is that it is based on atomic numbers which is more fundamental property than atomic weight. Electronic configuration of an element defines the position of an element in the periodic table. The long form of the periodic table makes it easy to view the regular changes in the properties of various element when we move across a period or down a group. The few problems with the periodic table is that the position of hydrogen is ambiguous as its properties relate to two groups and that the long form of periodic table does not contain inner transition elements in the main body of the periodic table. The long form of the periodic table has few contentions. As new elements and their properties are discovered sometimes certain elements exhibit ambiguous chemical behavior.