Different Types of Chemical bonding in Molecules
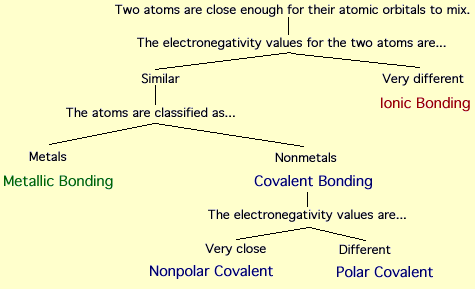
In molecules, chemical bonding can be classified into four types
1) ionic bonding
2) covalent bonding
3) Metallic bonding
4) Vander Waal's bonding
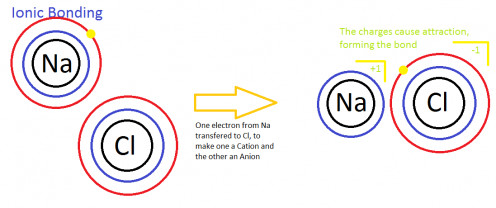
Ionic bonding
This type of bod is formed as a result of complete transfer of electrons from an electro positive element to an electro negative element, thereby creating positive and ionic ions.
Example: NaCl
Ionic crystals are brittle, soft and poor conductors of heat and electricity. They have high melting points and high latent heat of fusion.
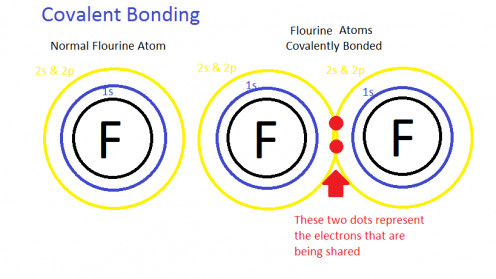
Covalent bonding
It is formed by the sharing of electrons between two identical atoms so as to obtain a stable configuration.
Example: Formation of H2 molecule. Such molecules are very hard and pure conductors of electricity. They have high melting point and high latent heat of fusion.
Example: Bonding in silicon, quartz, diamond
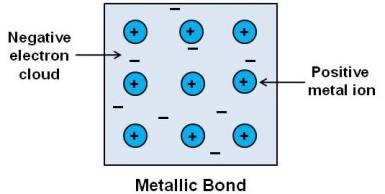
Metallic bonding
Metals consists of positive ion cores immersed in a gas of free electrons. The force of attraction between the electron gas and metal ions hold the metal together. This force of attraction is called metallic bond. Metallic bonds are weaker than ionic or covalent bonds.
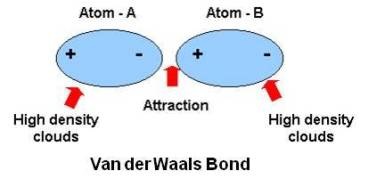
Vander Waal's bonding
Inert gases (He, Ne etc) condense into solids at very low temperatures. This is possible if there exists the force of attraction called Vander Waal's forces. They are weak binding forces. Molecular solids such as CH4, Cl2, I2, and CO2 etc. formed due to Vander Waal's interaction.
Chemical Bonding: An overview