Electron-Pair Repulsion Theory
What is it?
- The Electron-Pair Repulsion Theory is basically a theory that states that the valence electron pairs surrounding an atom will mutually repel each other and thus determine the molecular geometry or shape of the molecule itself.
- From this theory you can predict the shape of a molecule based on the extent of the electrostatic repulsion of the electron pairs.
How does it work?
Imagine trying to force the same poles of two magnets together, what would happen? They would repel each other! This is much the same for electrons, because they are negatively charged the electron pair in the outer shell of the central atom of a molecule will repel the other electron pairs in the other atoms of the molecule.
This repulsion will cause the electron pairs to move as far away from each other as possible, whilst keeping within the confines of the intermolecular bonds that are holding the molecule together.
The molecule will then form a 3-Dimensional shape because of this.
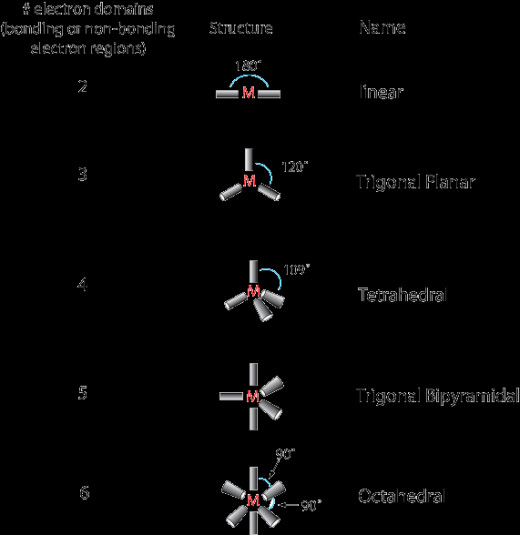
Below are the main shapes that molecules can form.
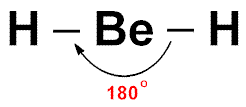
Linear Molecules
- An example of a linear molecule (as seen in the picture to the right) is Beryllium Hydride (BeH2).
- Linear molecules have 2 bonded pairs of electrons and no lone pairs.
- The bonds will repel as far as possible and create a molecule with a bond angle of 180o.
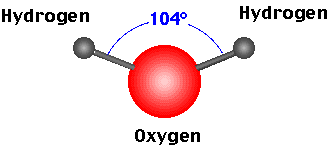
Non-Linear Molecules
- An example of a Non-Linear molecule is H2O (water).
- Because there are 6 outermost electrons in an oxygen atom and only 1 outermost electron in each hydrogen atom, the H2O molecule will have 2 bonded pairs of electrons and 2 lone pairs of electrons.
- The lone pairs will repel more than the bonded pairs of electrons and therefore repel the other bonded electron pairs further away from itself.
- This repulsion creates a Non-Linear (or 'bent') shape with bond angles of 104o.

Trigonal Planar Shapes
- An example of a molecule with a trigonal planar shape is Boron Triflouride (BF3).
- Boron Triflouride has 3 bonded pairs of electrons and no lone pairs.
- This molecule is very reactive and will accept lone pairs of electrons.
- Because of the equal repulsion between the atoms, Boron Triflouride forms a Trigonal Planar shape with a bond angle of 120o.

Trigonol Bipyramidal Shape
- An example of a molecule with a Trigonol Bipyramidal shape is Ammonia (NH3).
- Ammonia has three bonding pairs and one lone pair of electrons on the central nitrogen atom.
- The lone pair decreases the bond angle of the atom because it repels more than the bonded pairs.
- This gives the Ammonia molecule a Trigonol Bipyramidal shape with a bond angle of 107o.
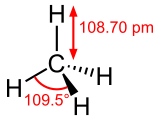
Tetrahedral Shape
- Methane (CH4) is an example of a molecule with a tetrahedral shape.
- There are 4 bonding pairs of electrons between the atoms and no lone pairs.
- These pairs repel each other to the corners of a regular tetrahedron.
- This creates the tetrahedral shape of the molecule, with a bond angle of 109.5o.
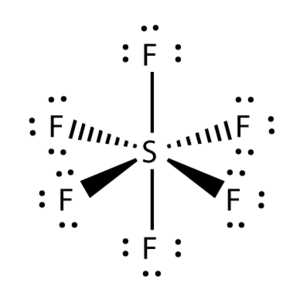
Octahedron Shape
- An example of a molecule with an octahedron shape is Sulfur Hexaflouride (SF6).
- It has 6 bonded pairs and no lone pairs.
- All of the bonded pairs in Sulfur Hexaflouride repel equally.
- The bond angles in an octahedron shaped moleculeare 90o.