Electronegativity
Electronegativity refers to the ability of an atom to attract electrons that it is sharing with another atom.
The trend in electronegativity generally follows that of ionization energy and the reason for the trend is pretty much the same. Across a period (horizontal row), electronegativity increases from left to right, and within a group (vertical column), electronegativity decreases. We can also say that the trend in electronegativity is increasing towards the upper right-hand corner of the periodic table. Thus, fluorine (F) is the most electronegative element. Note that, based on the trend, Ne and He would be expected to be more electronegative than F. However, in nature, He, Ne and the other noble gases are not found sharing electrons with other atoms; they are usually ignored when discussing electronegativities.
Example: which is more electronegative: a metal or a nonmetal?
Answer: Nonmetals are more electronegative than nonmetals. Nonmetals are found on the upper right-hand corner of the periodic table.
The Pauling Scale
On the Pauling scale, atoms are assigned electronegativities ranging from 0.7 for the least electronegative atom (Francium) to 4.0 for the most electronegative atom (Fluorine); on this scale, hydrogen has an electronegativity of 2.2. Some of Pauling's original values for the most electronegative elements are given below:
F (4.0), O (3.5), N and Cl (3.0),
Br (2.8), S and C (2.5), I and Se (2.4),
P, H, and The (2.1)
Pauling later changed the value for H to 2.2. The values for the least electronegative (most "electropositive") elements are:
Cs (0.7), Rb and K (0.8), Na and B (0.9), Li, Ca and Sr (1.0), Mg (1.2), Be and Al (1.5)
For an interesting account of how Linus Pauling came up with the numbers, Click Here.
There are several other scales that have been suggested, including some that slightly modify Pauling's scale; Click Here.
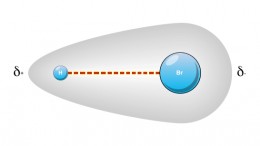
Bond Polarity
With the electronegativity difference between F and the least electronegative nonmetals being around 1.8 or 1.9, we can pretty much consider any pair of atoms that differ in electronegativity by 2.0 or higher as being in an ionic bond. Pauling defined the percent ionic character of a bond using the equation:
%ionic character = 1-exp(-x2/4),
where x is the electronegativity difference between the two atoms. Plugging in a value of 2.0 for x gives us
%ionic character = 1-exp(-2.02/4) = 0.63, or 63%
If we try to calculate the electronegativity difference between pairs of atoms, we will find that some pairs of atoms that we would normally consider to form ionic bonds (metal + nonmetal) actually have an electronegativity difference less than 2.0. These bonds between these atoms should be considered as polar covalent bonds, not ionic. Indeed, the "metal + nonmetal = ionic bond" rule is just a rule of thumb. There are a lot a of exceptions. Indeed, an entire field of study (organometallic chemistry) deals with covalently bonded transition metal atoms.
If the electronegativity difference between two atoms is larger than zero but less than 0.5, the polarization is negligible; the bond between them is classified aspolar covalent, but essentially nonpolar.
Example: classify the bond between C and H, Li and I, Na and Cl, Na and Na
Answer:
For C and H: electronegativities of C and H are 2.5 and 2.2; difference = 0.3. Bond is polar covalent, but essentially nonpolar.
For Li and I: electronegativities of Li and I are 1.0 and 2.4; difference = 1.4. The bond is polar covalent.
For Na and Cl: electronegativities of Na and Cl are 0.9 and 3.0; difference = 2.1. The bond is ionic.
For Na and Na: the electronegativity difference is obviously zero; bond is pure covalent
[Note: metallic bonding refers to bonding among atoms in a metallic solid; but the bond between just two atoms of a metallic element is pure covalent].
© 2015 Discover the World



