A Brief Study Guide of Energy in Physics
Introduction
Energy in physics is defined as an object’s ability to do work through a distance. This is illustrated with the equation of E=W and W=Fd, where E is energy, W is work, F is force, and d is distance. There are different forms of energy such as kinetic, gravitational potential, spring potential, and internal energy. Energy can be transferred through and to objects by different means, but will always follow the Law of Conservation of Energy.
Kinetic and Gravitational Potential Energy
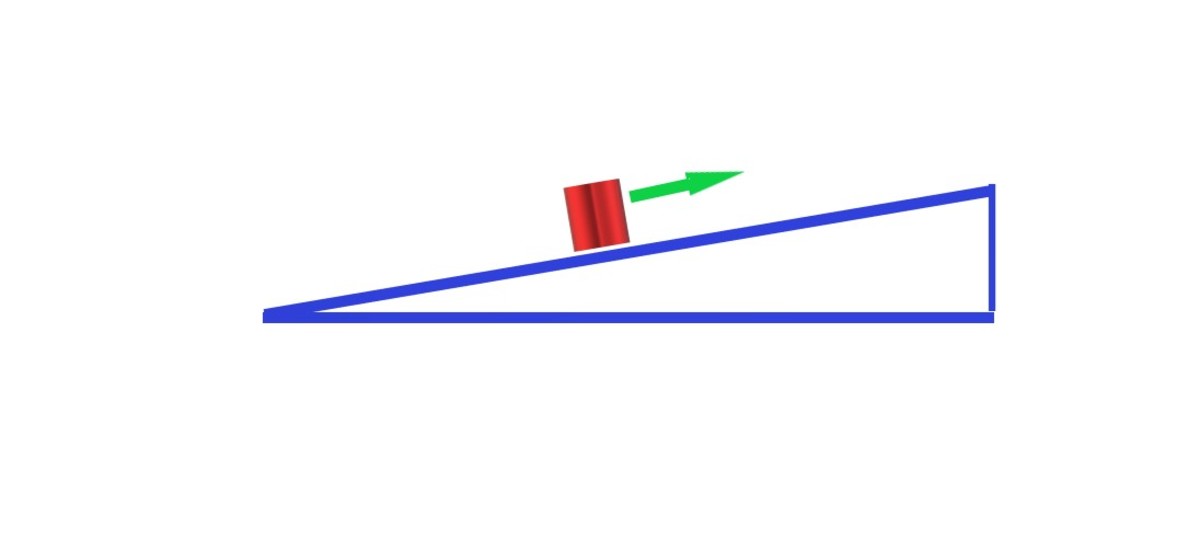
The first type of energy is kinetic energy which is the work done to set an object in motion, or the energy of motion. This is illustrated by the equation: KE=1/2mv^2 with m being the mass and v being the velocity. The next type of energy is Gravitational Potential Energy, which is the amount of work done to life an object that is subjected to gravity. Gravitational potential energy can also refer to the energy of the position of an object. This is illustrated by the equation GPE=mgh, where m is the mass, g is the acceleration of gravity which is 9.8 m/s^2, and h is the height of the object. Kinetic energy and gravitational potential energy together make up what is known as total energy, or just energy. Total energy is displayed by the equation E=1/2mv^2 + mgh, or simply the kinetic energy plus the gravitational potential energy.
Kinetic Energy
| Gravitational Potential Energy
| Total Energy
|
|---|---|---|
KE=1/2mv^2
| GPE=mgh
| E=1/2mv^2+mgh
|
Energy of an object in motion
| Work needed to lift an object
| KE+GPE
|
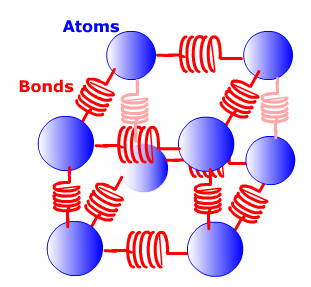
Spring Potential Energy and Internal Energy
Another form of energy is known as spring potential energy, which is the energy in compressing and releasing a spring. The equation SPE=1/2Kd^2, where K is the spring constant or stiffness of the spring, shows how force is exerted through the spring, causing work to be done. The last form of energy is internal energy. Internal energy refers to the total kinetic energy of all the atoms and the total spring potential energy of all the bonds in a system. As atoms move they exert kinetic energy and as they press against the bonds between atoms they use spring potential energy.
Transformation vs Transfer
There are two ways energy in a system can change or move. The first is energy transformation, or the process where energy stays constant. Since total energy is the kinetic energy plus the gravitational potential energy, as one increases or decreases the other value corresponds so that the total energy remains the same. An example could be a ball rolling up a hill; as the ball goes up the hill it begins to lose velocity due to gravity, but because the height is increasing, thus causing the gravitational force to increase, whatever energy is lost in the kinetic energy is made up by the gravitational potential energy. This specifically follows the Law of Conservation of Energy, which states: If gravity is the only force (no air resistance, friction, etc.), then energy will stay constant during motion. The example of the ball rolling up a hill shows how the energy stays constant even while values such as kinetic energy change.
The last way energy can change in a system is through energy transfer. Energy transfer is the process where the energy of a system changes to another. Some examples are heat, sound, and work. Specifically for heat the transfer is obvious. For example the energy from an oven causes it to warm up, which transfers the energy from the oven to the now heated food.
Recap
In conclusion, energy is a basic principle in physics that has different forms and methods of changing, but ultimately all relate and work together in some way under the Law of Conservation of Energy. While energy may be lost in one form it could be gained in another so that the total energy level never changes (As a ball rolls down a hill its potential energy decreases as its kinetic energy increases equally, so the total energy never changes). Energy as a broad term can be broken down into smaller units that all function together to act as the property of work being done, also known as energy.