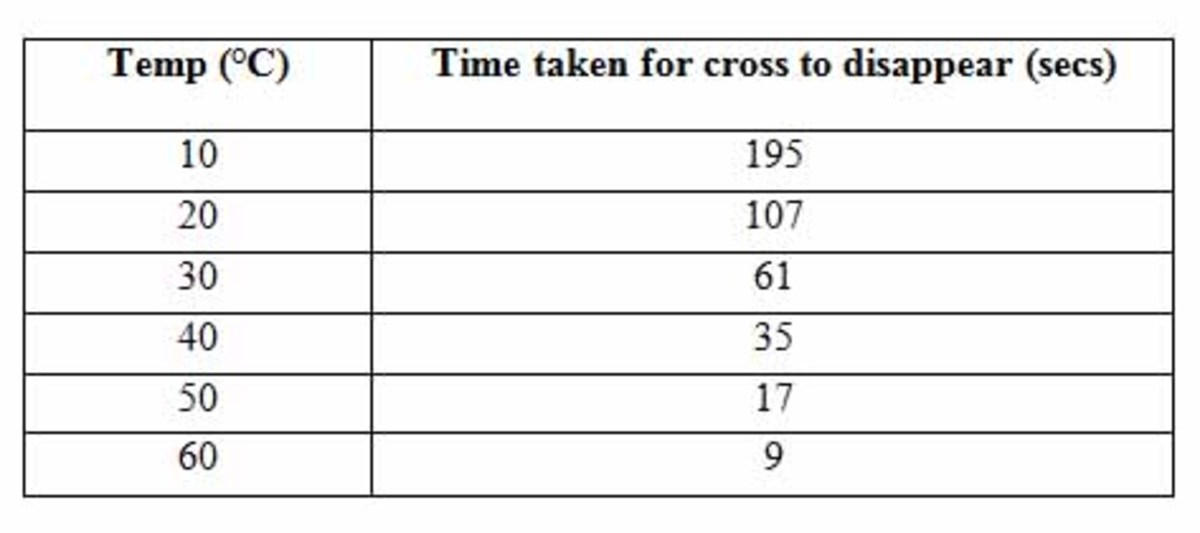
Fission-Fusion Reactor
Fission-fusion reactor is a device which involves splitting and combining of nuclei of atoms. In this reactor, nuclear fission and fusion reaction are takes place.It can be generates unlimited and constant energy. The reactor is divided into two parts. they are
- Fission Part
- Fusion part
Fission Part:
Fission reaction is a defined as splitting of nucleus by neutron which contains high speed in this part. It is involves chain reaction. One neutron bombarded to heavy nucleus and to forms two small nuclei and also forms some energy. It is coated over a metal is beryllium and base metal taken as Iridium for maintain the loses of heat.
The by-products include free neutrons, photons usually in the form gamma rays, and other nuclear fragments such as beta particles and alpha particles.
Fission of heavy elements is an exothermic reaction and can release substantial amounts of useful energy both as gamma rays and as kinetic energy of the fragments (heating the bulk material where fission takes place).

The most common fission process is binary fission, and it produces the fission products noted above, at 95±15 and 135±15 u. However, the binary process happens merely because it is the most probable. In anywhere from 2 to 4 fissions per 1000 in a nuclear reactor, a process called ternary fission produces three positively charged fragments (plus neutrons) and the smallest of these may range from so small a charge and mass as a proton (Z=1), to as large a fragment as argon (Z=18).
The most common small fragments are composed of 90% helium-4 nuclei with more energy than alpha particles from alpha decay (so-called "long range alphas" at ~ 16 MeV), plus helium-6 nuclei, and tritons (the nuclei of tritium). The ternary process is less common, but still ends up producing significant helium-4 and tritium gas buildup in the fuel rods of modern nuclear reactors.
Fusion part:
Fusion reaction is defined as combining of small nuclei at high temperature and high density to form heavy nucleus. It prevents chain reaction. This reaction involves at high temperature and high density.
It is the reaction in which two atoms of small nuclei combine together, or fuse, to form an atom of large nucleus. In the process some of the mass of the nucleus is converted into energy. For example, the reactions occurs in sun.
The easiest fusion reaction to make happen is combining deuterium (or “heavy hydrogen) with tritium (or “heavy-heavy hydrogen”) to make helium and a neutron. Deuterium is plentifully available in ordinary water. Tritium can be produced by combining the fusion neutron with the abundant light metal lithium.
Thus fusion has the potential to be an inexhaustible source of energy. This part is to be maintain the high temperature by carbon dioxide or other gases in between the inside and outside of the reactor.
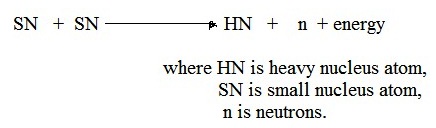
Energy released in most nuclear reactions is much larger than in chemical reactions, because the binding energy that holds a nucleus together is far greater than the energy that holds electrons to a nucleus.
For example, the ionization energy gained by adding an electron to a hydrogen nucleus is 13.6 eV—less than one-millionth of the 17.6 MeV released in the deuterium–tritium (D–T) reaction shown in the diagram to the right (one gram of matter would release 339 GJ of energy).
Fusion reactions have an energy density many times greater than nuclear fission; the reactions produce far greater energy per unit of mass even though individual fission reactions are generally much more energetic than individual fusion ones, which are themselves millions of times more energetic than chemical reactions.
Only direct conversion of mass into energy, such as that caused by the annihilatory collision of matter and antimatter, is more energetic per unit of mass than nuclear fusion.
Difference between Fission and Fusion Reactions:
Fission Reaction
| Fusion Reaction
|
|---|---|
Splitting of nucleus
| Combining of nucleus
|
Fission reaction does not normally occur in nature.
| Fusion occurs in stars, such as the sun.
|
Fission produces many highly radioactive particles
| Few radioactive particles are produced by fusion reaction, but if a fission "trigger" is used, radioactive particles will result from that.
|
Critical mass of the substance and high-speed neutrons are required.
| High density, high temperature environment required.
|
Takes little energy to split two atoms in a fission reaction.
| Extremely high energy is required to bring two or more protons close overcome their electrostatic repulsion.
|
The energy released by fission is million times greater than that released in chemical reactions, but lower than the energy released by nuclear fusion.
| The energy released by fusion is three to four times greater than the energy released by fission.
|
It gains mass of atoms at end of fission reaction.
| It loses mass of atoms at end of fusion reaction.
|
Activity
Aim:
To generate constant unlimited energy from uranium by fission-fusion Reactor.
Apparatus:
Fission-Fusion reactor, Generator which install with dynamo.
Required chemicals:
Uranium oxide fuel, or other radio active elements ore.
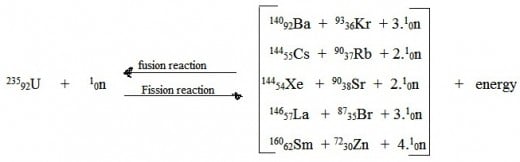
Procedure:
- Take uranium oxide fuel and sent into reactor at fission part.
- After that we have to burn uranium oxide in reactor.
- Later uranium atom splinted into fission fragments, neutrons and some energy.
- Those fission fragments sent into fusion part of reactor.
- In fusion part of reactor, fission fragments combined and forms uranium atom due to attraction between protons and neutrons.
- During fusion reaction f uranium atom, repulison of same charged particles causes gernerate of energy.
- Finally Uranium atom splinted and combined in Fission-Fusion Reactor.
- Released energy was convert into electrical energy by using dynamo
- So water sent into tubes in reactor then water converted into steam.
- Those steam sent through turbines to run dynamo for generate of electrical energy.
Result:
In reactor, uranium atoms invovles fission and fusion reaction, and gives constant and unlimited energy.
© 2014 KALYAN CHAKRAVARTHY THADAKA