GCSE Chemistry Revision - Moles

Definition
A mole is a word that represents a number, much like the word 'dozen' represents the number 12, a mole represents the number 6x1023 (this number is also known as Avogadro's number).
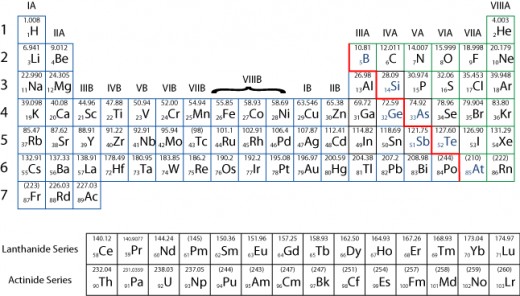
On the periodic table the relative atomic mass number of carbon is 12 (12C or 'carbon 12')
A mole is the amount of atoms in 12g of carbon 12, so one mole of carbon atoms has a mass of 12g.
Just like one mole of carbon atoms has a mass of 12g, one mole of the atoms of any element is equal to it's relative atomic mass in grams (the relative formula mass number is the number above the element on the periodic table).
For example, the relative atomic mass of magnesium is 24 therefore one mole of magnesium is 24g. We can then use this information to work out the amount of moles in molecules or compounds.
Hydrogen exists as a diatomic molecule, meaning it is a molecule that contains 2 atoms (H2). So, one mole of hydrogen would be 2 times it's relative atomic mass (which is 1) so one moles of hydrogen would be 2g. This is called the relative molecular mass.
In order to find the mass of one mole of a compound you add up all of the relative atomic masses (RAM's) and the number that you get is called the relative formula mass (RFM).
For example, to find one mass mole of carbon dioxide (CO2) you add up all of the RAM's (Carbon = 12 Oxygen = 16). 12 + (16 x 2) = 44, so one mole of carbon dioxide (the relative molecular mass) is 44g.
The equation that converts mass into moles is: moles = mass ÷ RFM (relative formula mass)