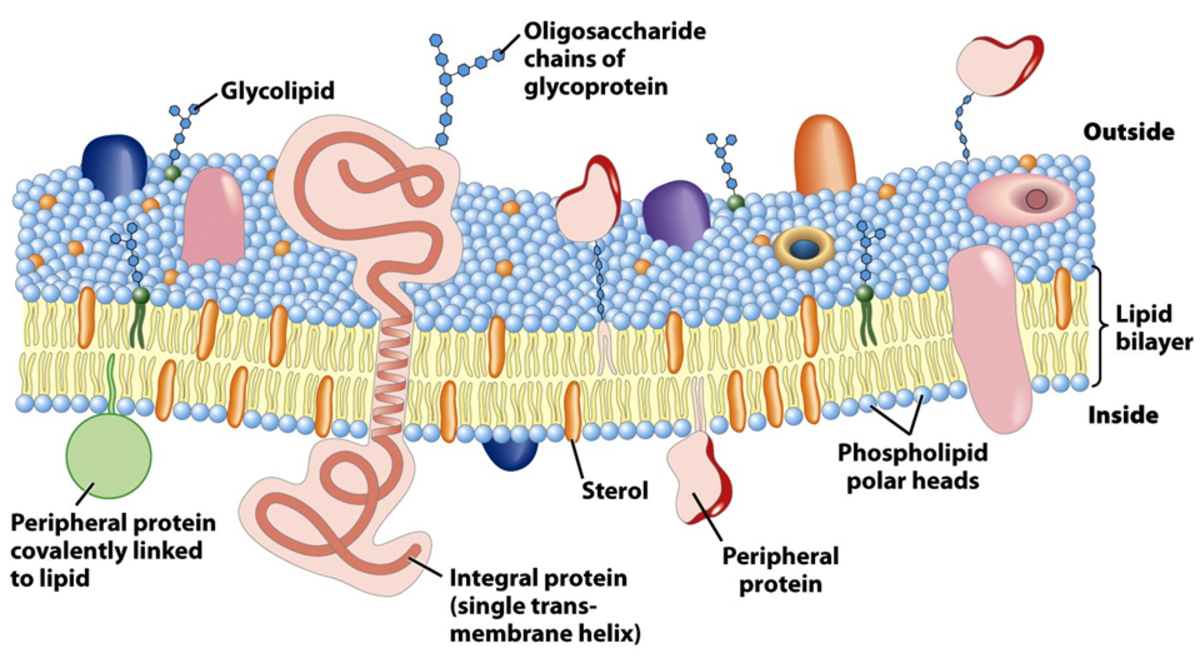
What is Glycophorin?
Glycophorin:
On the outside has glycosylation sites that are made of G which are the glycosylation sites while inside are made of polar, charged residues. The inside are made of hydrophobic regions. Glycophorin are made in the erythrocyte membrane proteins. It's single polypeptide are made of 131 aa. It's outside surface are negatively charged carbohydrate. Erythrocytes has hydrophilic, anionic outer surface and this is useful because ......Bacteriorhodopsin is used in the production of ATP for transporting protons out the cell using light energy. Within the helical structure is buried a molecule of retinal; this retinal structure changes confirmation when a proton is absorbed results in change in confirmation of protein-proton transport. Bacteriorhodopsin are made of helices that cross the lipid bilayer membrane. Almost all of the amino acids in the transmembrane are in the transmembrane helices. The interconnecting loops are very short that connect the helices. The alpha helices are the backbones of the Bacteriorhodopsin that are completely hydrogen bonded. The hydrophilic residues are either in the ends or in the centre of the protein. The transmembrane proteins are the globular protein that part of the protein that crosses the transmembrane are hydrophobic. The structure of the membrane proteins are made of porins. The porins are beta sheets that crossing the membrane. The outside is non-polar, while the inside is hydrophilic and contains water. Porin is the transmembrane protein and water. A very little water is here.....(diag). The water is distributed around the protein. Some proteins are partly embedded in the membrane. Prostaglandin H2 Synthase converts arachidonic acid into Prostaglandin H2. alpha helices are embedded in the membrane. Prostaglandin H2 synthase is an integral membrane protein. Why is it useful to have this protein embedded in the membrane. Receptor Tyrosine KinasesBiologically very important class of membrane proteins. It is key role in many process-including diseases. There are many different classes known including EGF, insulin receptor(diag). Some exist as monomer others as multimers. Intial event in receptor tyrosine kinase signalling: extracellular ligand binds resulting in oligomerisation, intracellular tyrosine kinase domains phosphorylate each other, generating a binding site for SH2-containing adaptor proteins such as Grb2(diag). Receptor tyrosine kinases: Epidermal growth factor (EGFR) HER2 (diag) structurally only available for extracellular domain, has 4 subdomains, and 11 activating growth factors identified for ErbB receptors. membrane proteins either integral or peripheral. location of a transmembrane segment in a sequence can be predicted. Movement across the membrane, factors affecting transport .......Lipid soluble molecules can pass through the membrane,