History of Atomic Theory

Atomism
The word atom literally means uncut (a = not, tomos = cut). The philosophy of atomism, espoused by the Greek philosopher Democritus (460 BC-370 BC), asserts that the world is made up of two things: atoms and the void. In other words, matter of made up of tiny, indestructible particle and in between atoms, there is nothing. However, atomism did not fluorish as it was contrary to the ideas supported by more influential people of that time (Plato, Aristotle, and the early Church). Furthermore, the Greek philosphers were thinkers, not workers. They did not have any experimental support for the ideas.
The scientific method as we know it, the use of empirical (experimental) data to support ideas, was not developed until centuries later. The first publication regarding the "proper way" to do science is credited to Sir Francis Bacon (1620). Before that, alchemists (early chemists) developed laboratory techniques that were eventually used for scientific work. The alchemists' goals were to turn lead into gold, cure diseases with herbs, develop the elixir of life (panacea) and develop a universal solvent that can dissolve anything ("alkahest"). Their approach to achieving their goals, however, is not what we would consider as "scientific". Although alchemists did not advance our knowledge about atoms, they promoted observation and experimentation and served as bridge from Greek philosophers’ “pure thought” and modern scientific method.
John Dalton elevated the idea of atomism to a scientific theory in 1803. In Dalton's atomic theory, the following assumptions (postulates) are made:
- matter consists of small indivisible particles called atoms
- an element consists of identical atoms (same mass)
- atoms of different elements have different masses
- atoms of two or more elements combine in small whole-number ratios to form compounds
- a chemical change involves the rearrangement of atoms
Extensive work by several scientists provided the empirical support for Dalton's ideas. Among the most notable are the work of Antoine Lavoisier (the "father" of Modern Chemistry) and Joseph Proust.
Lavoisier's work led to the formulation of the Law of Conservation of Mass, which says that the amount of matter is constant even during a chemical change. Dalton's assumption that atoms are indestructible and merely rearrange during chemical changes explains this general observation. Here's an animation showing the rearrangement of atoms when hydrogen and oxygen react to form water. Click on "magnify the reaction" to see the atomic representation.
Proust's work led to the formulation of the Law of Definite Proportions, which says that there are substances, called compounds, made up of more than one element that have a well-defined composition. By this, we mean that the mass-to-mass ratio of any two elements in the compound is the same for any sample of the compound. Dalton's theory explains that this would be a consequence of the assumptions that all atoms of an element have the same mass and the small whole-number ratio (of atoms from different elements) is well defined for a specific compound. To illustrate, suppose a compound is made up of elements X and Y. Suppose the formula of the compoud is XY2. This means that the X:Y atom ratio is 1:2; that is, for every atom of X in the compound, there are also two atoms of Y in the compound. Also suppose that each X atom has a mass of 16 units, and each Y atom has a mass of 1 unit. The mass of one X atom is (1)(16) or 16 units; the mass of two Y atoms is (2)(1) or 2 units. Therefore, for any sample of this compound, the mass ratio of X to Y is 16:2, or 8:1.
Dalton himself formulated the Law of Multiple Proportion, which says that if two elements can form more than one compound, the ratio of their mass ratios can be reduced to small whole number ratio. To illustrate, suppose elements X and Y above can also form a compound with a formula of XY. Then the mass ratio of X to Y for this compound would be 16:1. The ratio of mass-to-mass ratios would be (8/1) : (16/1), which reduces to 1:2.
For an excellent website dealing with how the idea of atoms began, Click here
Be sure to play in Dalton's playhouse.
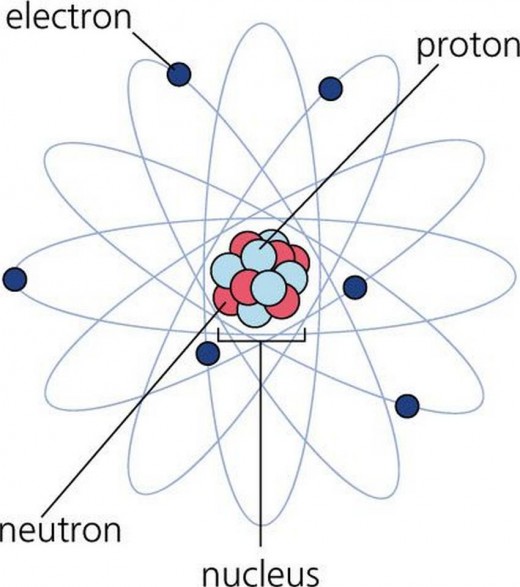

The Discovery of The Electron
Theories evolve as we discover new information. Some of Dalton's assumptions about atoms are no longer accepted. Atoms are, in fact, divisible. We now know that they are made up of even smaller particles called protons, neutrons, and electrons. The discovery of the electron is credited to Joseph John Thomson, although our experience with it dates back to the time of Thales of Miletus (600 BC), where it was found that rubbing amber against fur makes them attract each other. We now interpret this phenomenon in terms of electrons being transferred from fur to amber. The fur becomes positively-charged and the amber becomes negatively-charged. In 1600, William Gilbert discovered that amber was not the only material that can attract other materials when rubbed; he coined the word electric (from elektron, the Greek word for amber) and referred to materials that attract each other when rubbed as electrically charged. In 1629, Niccolo Cabeo discovered that electrically charged materials can also repel each other. This led to the notion of two kinds of charge; like charged materials repel each other, materials with unlike charge attract each other. In 1733, Charles du Fay proposed two kinds of charge: resinous and vitreous. By his definition, vitreous charges are transferred to a glass rod when it is rubbed against silk, and resinous charges are transferred to amber when it is rubbed against fur. We now know that what du Fay called resinous charges are negatively charged electrons. We also now know that when glass is rubbed against silk, it becomes positively charged, not because positively charged particles (vitreous charge) are transferred to the glass but because electrons moved out of the glass. The idea of just one type of charged particle being transferred is credited to Benjamin Franklin (1752), although he assumed that the charge that is transferred is vitreous (positive). Measurement of charges is attributed to Charles Augustin de Coulomb who formulated (in 1785) the law that now bears his name : the force between two charged particles is directly proportional to the product of the magnitudes of the charge and inversely proportional to the square of the distance between them. The SI unit for electrical charge is C (Coulomb).
J. J. Thomson used cathode ray tubes (CRT) in his experiments (1897). Inside a CRT, a piece of metal (the cathode) is subjected to high voltage. As a result, a beam of particles is ejected from the metal. We now know that the particles in the beam are electrons. J. J. Thomson measured the charge-to-mass ratio for the particles. Robert Millikan's oil-drop experiment (1909) established the charge of the electron. In his experiments, Millikan found that when he charged oil droplets, the charges were all multiples of -1.6x10-19 C. This suggested that -1.6x10-19 C is the smallest possible charge and this is the charge carried by each electron. Based on J. J. Thomson's measured charge-to-mass ratio and Millikan's measured charge, the mass of the electron was found to be 9.1x10-31 kg, much smaller than the lightest atom known (hydrogen). Moreover, beams behaved the same way (when subjected to magnetic or electric fields) in J. J. Thomson's experiments regardless of the nature of material used as cathode. Therefore, it was concluded that whatever these particles are, they are found in all atoms.
For an interesting account of J. J. Thomson's work, read here. or watch this YouTube video (start at 3:20):
A simulation of Millikan's experiment can be found Here.
With the discovery of the electron, the plum pudding model was proposed for the atom. Instead of structureless, indivisible spheres that Dalton envisioned, J. J. Thomson proposed that atoms are positively charged spheres with negatively charged electrons embedded in them, just like plums in a pudding, Click here.

The Discovery of The Nucleus
The work of Ernest Rutherfordprovided the next major advancement in our knowledge about atoms. In his experiments (1909), Rutherford bombarded a piece of gold foil by alpha particles. Alpha particles are high speed particles produced by radioactive materials and we now know that an alpha particle is a helium nucleus (2 protons and 2 neutrons). If the plum pudding model were correct where the mass of of an atom is spread evenly throughout the space occupied by the atom, the alpha particles would all be expected to pass through with very minor deflection (like bullets through a tissue paper). As it turned out, most of the alpha particles passed through the gold foil with very minor deflection, but a very small fraction of the particles were deflected at very large angles and bounced back ("backscattered"). This led to the proposition of a nuclear model for the atom, where:
- the mass of atom is concentrated in a very tiny spot (the nucleus),
- the nucleus is positively charged,
- electrons move around the nucleus, and
- the space where electrons move about is much much larger than the space occupied by the nucleus (the diameter of the region where electrons move is at least 1000x larger than the diameter of the nucleus)
There are numerous excellent resources on the web dealing with Rutherford's alpha scattering experiment.
- and watch this YouTube video:

The Discovery of The Neutron
The Discovery was made in 1932 by Sir James Chadwick, years after Rutherford discovered the nucleus. Neutrons were more difficult to detect because they are electrically neutral. Electrically charged particles like electrons and protons are easier to detect because they respond to magnetic and electric fields. For more information of the discovery:
© 2015 Discover the World