Homemade Fire Extinguisher: A Fun Children's Activity/Experiment
Background
Vinegar and baking soda, when combined, go through two reactions. The first is the reaction between acetic acid in vinegar and sodium bicarbonate in baking soda. When combined, they produce carbonic acid. Carbonic acid is unstable and therefore falls apart into two components: carbon dioxide and water. Carbon dioxide is what we breathe out. When all of this happens, pressure is built up.
What you will need:
A candle and matches
Jar with a metal lid
Hammer and punch (or nail)
A small container that can fit inside the jar and be able to tip over
Masking tape or a sharpie
Vinegar (amount based on size of jar)
Baking soda (amount based on size of small container)
Water
Experiment
1) Take the metal lid from the jar and make a hole in the center using the hammer and punch.
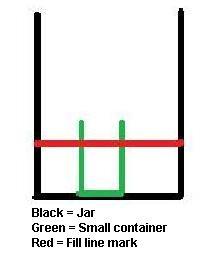
2) Place the small container in the jar and mark the outside of the jar using masking tape or a sharpie (masking tape works better). The mark should be just below the top of the small container (See diagram). This will be your fill line.
3) Remove the small container and fill the jar with vinegar to the mark you made.
4) Add baking soda to the small container and mix in just enough water to make it flow. (3 parts baking soda to 1 part water tends to be the ideal ratio)
5) Carefully place the container inside the jar, making sure not to spill any baking soda into the vinegar.
6) Gently screw the lid back onto the jar, once again being careful not to spill anything yet.
7) Light the candle.
*NOTE* The next part will be messy. It is best to do this outside or in a shower or tub.
8) Tilt the jar and point the hole towards the flame. The small container should spill into the vinegar. Once this happens the reaction will build pressure until liquid begins shooting out of the hole in the lid in a tight stream. Keep the stream pointed in a safe direction until the reaction is complete (the stream stops).