Chemistry for Kids: Learn About Atoms and Bonds
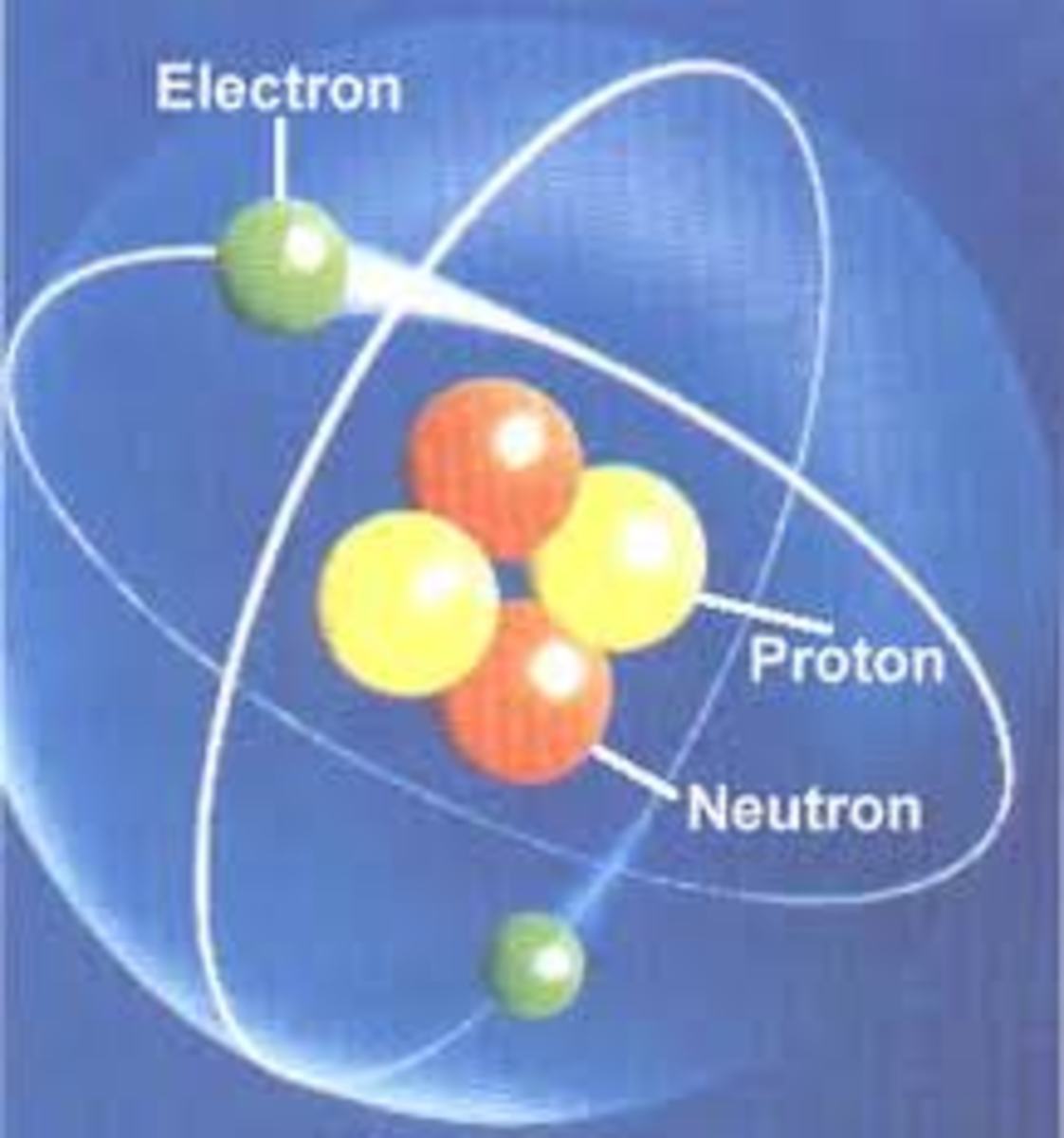
Atoms are the building blocks of everything. Yes, everything! They get together and make up something called matter. You're made of atoms. Your body's cells are made of atoms. Your toys are made of atoms. Your pets are made of atoms. Even the planet Earth is made up of atoms. They have three important parts: protons, neutrons and electrons.
They may be tiny but they’re very important. And when I say tiny, I mean tiny! You can’t even see them with a regular microscope. You need a special microscope called an electron microscope.
The word atom comes from the Greek word atomos, which means indivisible.
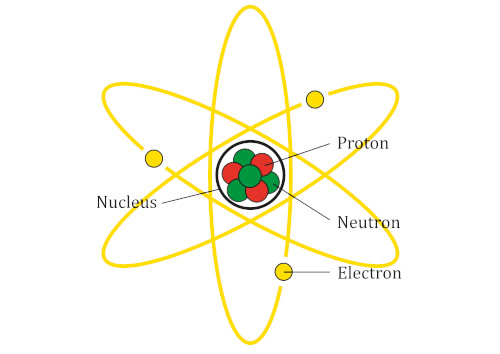
Structure of an Atom
The parts of an atom are:
- the nucleus
- protons
- neutrons
They are called subatomic particles.
The center of an atom is called the nucleus.
The nucleus is made up of protons and neutrons.
Electrons spin in orbits around the exterior of the nucleus.
Proton are positively charged, electrons are negatively charged, and neutrons have no charge.
Electrons move so fast that scientists have to make estimates of their locations.
Scientists believe that protons, neutrons, and electrons are made up of smaller substances called quarks and leptons.
Chemical Bonds
Remember that atoms are the building blocks of matter. But blocks are only useful if you can put them together. Just like with Lego blocks, atoms must be able to stick together. This is called bonding. A chemical bond is the force that holds atoms together in molecules.
There are different kinds of chemical bonds. They are:
- Ionic bond
- Covalent bond
- Metallic bond
Atoms use these different kinds of bonds to form molecules. They can also break bonds.
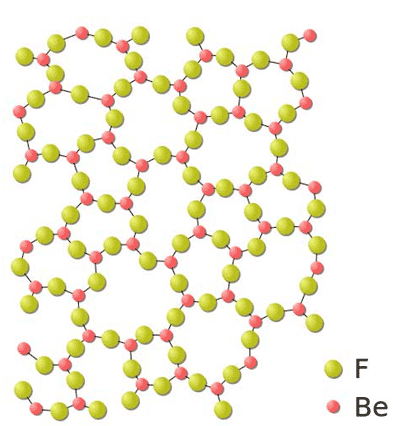
Example of a Chemical Bond
This is an example of a chemical bond involving two elements. F is flourine and Be is beryllium. When they bond they become BeF2 Beryllium Fluoride. Beryllium Fluoride is used to make a type of metal. Interestingly, flourine on it's own is a poisonous gas. Beryllium is a metal.
Atomic Number
The number of protons in the nucleus of an atom is called the atomic number. The atomic number determines the type of atom. Each hydrogen atom has one proton in its nucleus, so hydrogen has an atomic number of 1.
Each atom is associated with a specific chemical element. Atoms are the smallest units of an element. Each chemical element has a unique atomic number. Hydrogen is an example of a chemical element.
This content reflects the personal opinions of the author. It is accurate and true to the best of the author’s knowledge and should not be substituted for impartial fact or advice in legal, political, or personal matters.
© 2013 JoanCA