Light Fantastic
The Mystery of Light
Light is at once both obvious and mysterious. For some, looking at a rainbow is a beautiful and glorious creation to be admired. For others, there is a greater mystery longing to be answered. One that leads to an understanding of the bending and refraction of light. It’s this quest to understand the properties of light that has fundamentally transformed and shaped our world and has lead the way to the greatest scientific discovery of all time.
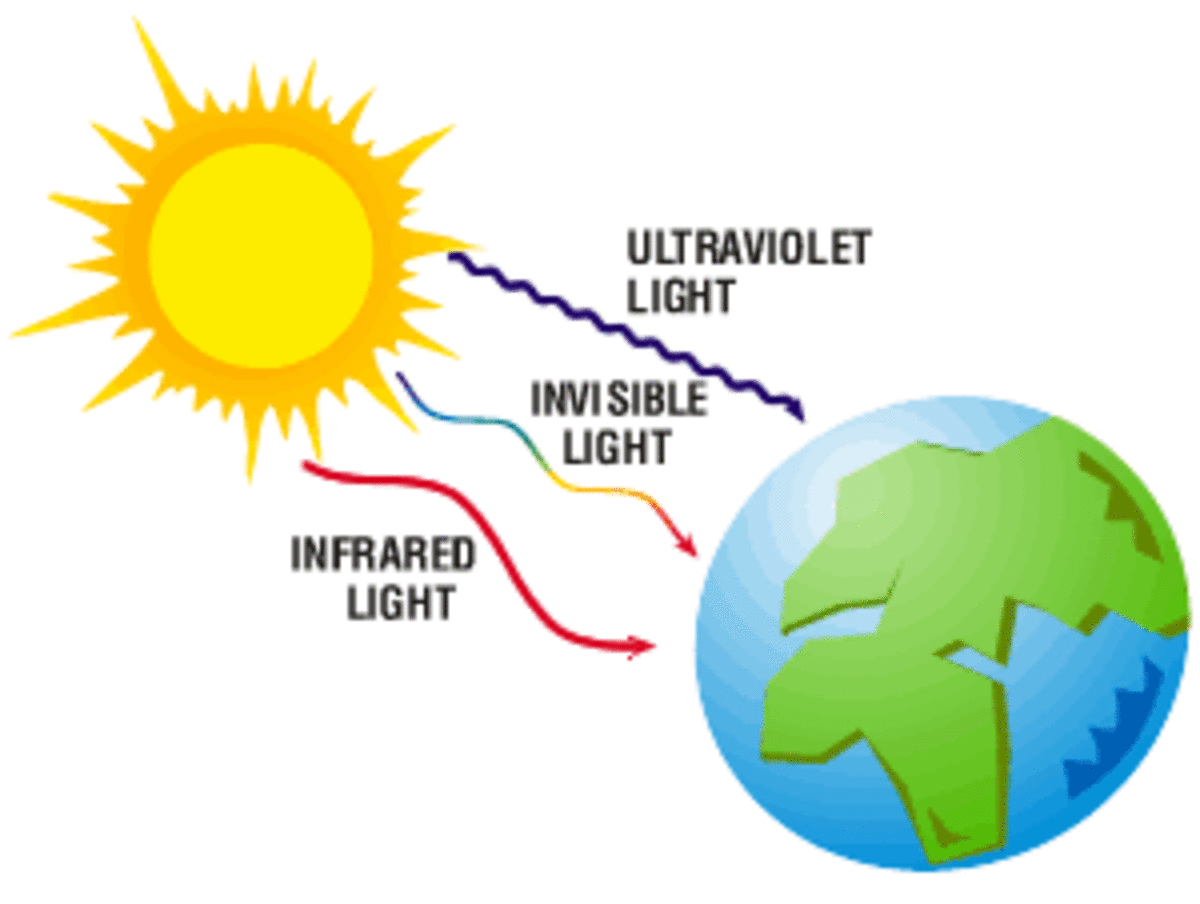
We know our Sun is life sustaining giving us heat energy and light. At a distance of approximately 93 million miles - a measurement that has only been known for the last 200 years – just how does all that light reach us? For one, it's hot - very hot. The Sun’s core is 15 million degrees Celsius while its surface is “only” about 5500° C. For another, it’s massive. If you could pour Earth sized marbles into its outer skin, you could fit 1.3 million of them. And the sun, mostly hydrogen gas (92%), produces the same energy as about a trillion one-megaton bombs – every second! And it’s been doing this for 4.5 billion years. It does this by a process called fusion – overcoming enormously strong atomic repelling forces to fuse hydrogen atoms into helium. It’s the energy released from this process that produces the light and heat that we get here on Earth. And there are other elements, approximately 60 others like beryllium and lithium. Just how, quite literally, on Earth we know the inner workings of the sun and the elements within came from our quest to understand optics and light.
Early Discoveries
Reason loves problems and can create questions where seemingly none exist. Some of the earliest philosophers and observers of nature where the ancient Greeks as they began to wonder just how optics worked and sought practical applications in navigation. In fact, the word optics comes from the Greek for “appearance.” It may seem obvious to us now, but back then, it wasn’t at all apparent that our own eye didn’t generate its own energy when cast upon an object. Many of these theories sought to describe light as a ray - a straight line moving from one point to another. Arab scholars took these ideas and honed them even further developing what is now known as geometrical optics - applying geometrical methods to the optics of lenses, mirrors and prisms. It wasn’t until the early 18th century that hard scientific evidence started to get some answers. Still, they were only beginning to guess the complicated physical and energetic properties at work that makes light possible.
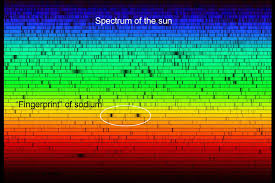
Absorption Sepctrum
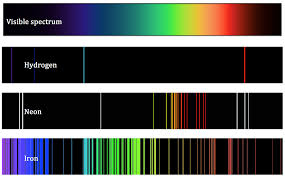
Emission Spectrum
The Spectrum of Light
Some notable early 18th century pioneers helped discover that certain gas and solid materials like hydrogen and helium absorb light particles from specific color ranges of the spectrum in a predictable and unique pattern. (See insert). And when these same elements were burned in a lab and the resulting light spectrum analyzed, the same pattern emerged as emission lines of light. The light from any source can therefore be analyzed to determine what elements are contained within it by taking note of these absorption and emission lines.
- Sir Isaac newton (1704) besides his work on gravity, Newton was the first to discover, using a prism, the diffusion of sun light into a visible rainbow of colors he called a spectrum. He proposed light as corpuscles, or particles. Newton also proved to his detractors that prisms do not alter light, they simply analyzed it as a tool.
- William Wollaston (1802) made the startling discovery that the spectrum of the Sun was not a solid band of color or continuous spectrum. He did this by looking at the spectrum of light under a magnifying lens. There he saw small black gaps or lines scattered throughout the spectrum. These small gaps would later be known as absorption lines.
- Gustav Kirchhoff (1859) was able to explain the basic rules for the creation of spectrum lines. Kirchhoff’s insight came when he began burning gases in the lab to observe the spectral lines they emitted. Some of these elements corresponded with same location of the missing black lines they had seen in Sun’s spectrum. It became apparent to scientists that each element had its own pattern of spectrum lines. These spectrum lines could be used, like a fingerprint, to identify elements. Throughout the 1860s, Kirchhoff managed to identify some 16 different chemical elements among the hundreds of lines he recorded in the sun's spectrum. From those data, Kirchhoff speculated on the sun's chemical composition as well as its structure. But, still none knew how the spectrum lines were created.
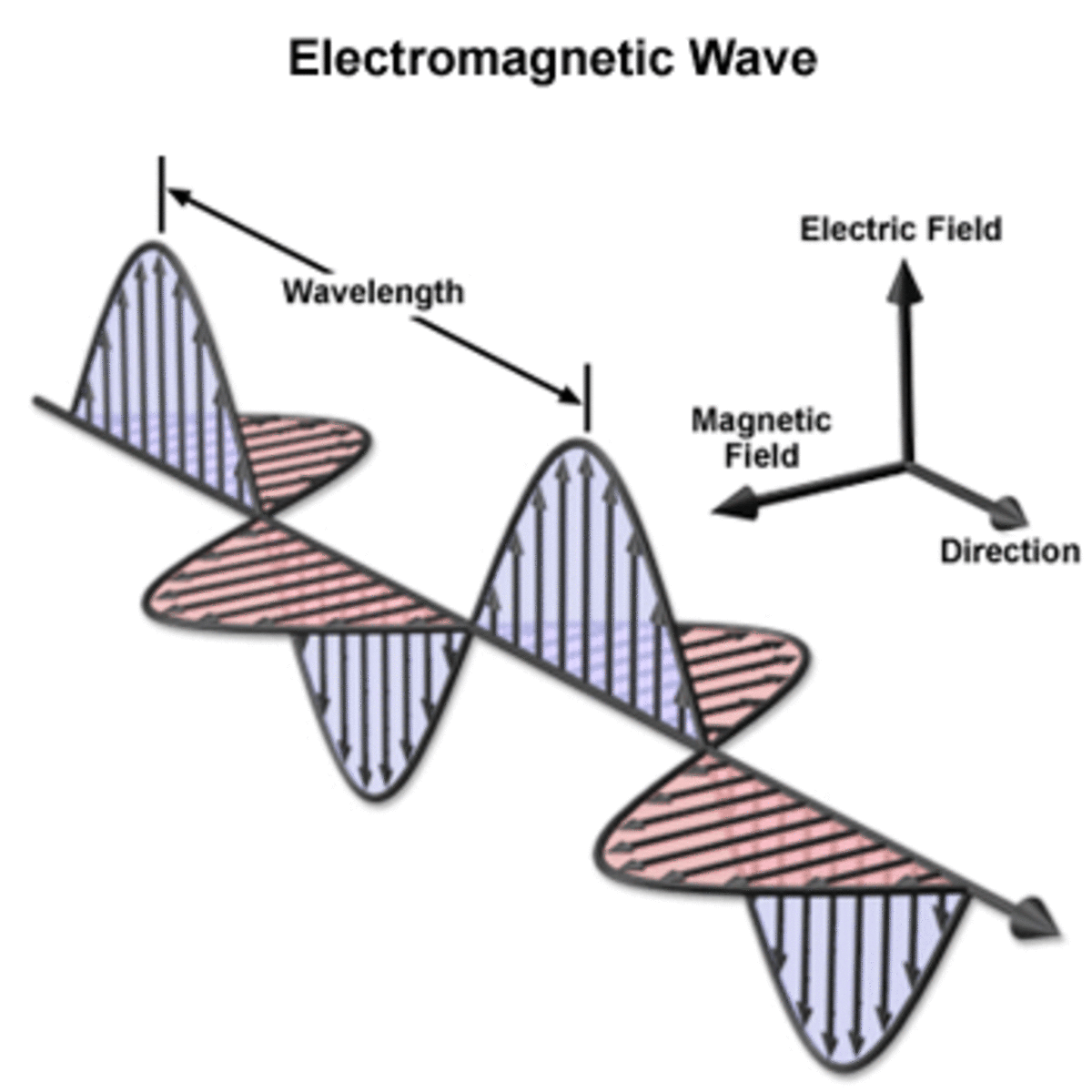
Maxwell's Equations - New Mathematical Language
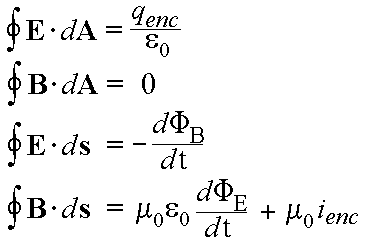
Light - An Electromagnetic Wave
Around this time, England was experiencing an industrial revolution. Many new discoveries in engineering and science where being made. Questions began to arise about magnetism, electricity and incandescent light. It was known that waving a magnet over copper coils would generate an electric current and in 1832, Michael Faraday produced a prototype electric motor. But he didn’t really understand why this machine worked? Enter James Maxwell.
James Maxwell in 1864 took the work of Faraday and others and used mathematics to investigate the fundamental causes of electrical and magnetic behavior, producing what are, to physicists today, some of the most beautiful equations created – Maxwell’s Equations. Maxwell proved that there must be electromagnetic waves, whose speed he was able to calculate. Maxwell’s calculated speed turned out to be identical to the speed of light, which people already knew from experiments. Maxwell then knew that light must be an electromagnetic wave. This was revolutionary. Maxwell was so advanced for his time, his contemporaries had no idea how he got to his conclusions - but his experiments proved correct. It would take brilliant physicists like Einstein and others to fully appreciate maxwell's work. And Maxwell's insights paved the way for other scientific discoveries.
Engergy, Frequency, Wavelength
Light Wave Lengths and Frequencies
Maxwell's understanding of electromagnetic waves made everything now make sense. They could now explain properties of light beyond the visible field that we could see with our eyes like ultra-violet light and it's affect on photo sensitive paper and x-rays.
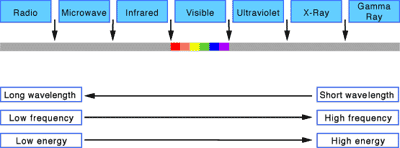
Scientists could now develop a complete working model of light using terms and concepts such as wavelength and frequency based on the structure waves. The frequency is the number of waves that pass a point in space during a time interval. We measure it in units of cycles (waves) per second. The frequency of visible light ranges from 430 trillion hertz in the red spectrum, to 750 trillion hertz in the violet spectrum. The full range of frequencies extends beyond the visible portion, from less than 3 billion hertz, as in radio waves, to billions of hertz as in gamma rays.
The amount of energy in a light wave is its frequency. High frequency light has high energy and low frequency light has low energy. Of visible light, violet has the most energy and red the least. The whole range of frequencies and energies is known as the electromagnetic spectrum (see image).
Light waves and Fequency
Light As Particles
Maxwell's theoretical treatment of electromagnetic radiation, including its description of light waves, was so elegant and predictive that many physicists in the 1890s thought that there was nothing more to say about light and how it worked.
- Max Planck, on Dec. 14, 1900, introduced a simple yet unsettling concept - light must carry energy in discrete quantities. Those quantities, he proposed, must be units of the basic energy increment he called hf where h is a universal constant (plank's constant) and f the frequency of radiation.
- Albert Einstein in 1905 using Planck's theory, studied the effect of shining ultraviolet light on the surface of a metal. When he did this, he was able to detect electrons being emitted from the surface. Einstein's theorized that If the energy in light comes in bundles, then one can think of light as containing tiny particles, or photons. When these photons strike a metal surface, they act like billiard balls, transferring their energy to electrons, which become dislodged from atoms.
This opened the debate again as light being a particle. Earlier scientists had demonstrated that atoms consist of positively charged nuclei surrounded by negatively charged electrons orbiting like planets, but they couldn't explain why electrons didn't simply spiral into the nucleus.
- Niels Bohr in1913 also used Planck's ideas to further develop the model of an atom. Bohr proposed that electrons exist in discrete orbits based on their energy. When an electron jumps from one orbit to a lower orbit, it gives off energy in the form of a photon. The quantum theory of light -- the idea that light exists as tiny packets, or particles, called photons -- slowly began to emerge. Our understanding of the physical world would no longer be the same.
Bohr's Model
Producing Photons
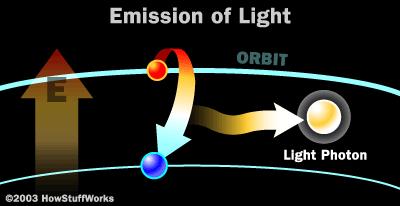
There are many different ways to produce photons, but all of them use the same mechanism inside an atom to do it. This involves energizing electrons orbiting an atomic nucleus. How Nuclear Radiation Works describes protons, neutrons and electrons in some detail. For example, hydrogen atoms have one electron orbiting the nucleus. Helium atoms have two electrons orbiting the nucleus. Aluminum atoms have 13 electrons circling the nucleus. Each atom has a preferred number of electrons zipping around its nucleus.
Electrons circle the nucleus in fixed orbits. There is a huge amount of theory around electron orbitals today, but essentially an electron has a natural orbit that it occupies. If you energize an atom, you can move its electrons to higher orbits. A photon is produced whenever an electron, stuck artificially in an orbit higher than normal, falls back to its original orbit. During this change from high to normal energy, the electron emits a photon of energy characteristics unique to it's atom's orbit. The photon has a frequency, or color, that exactly matches the distance the electron falls.
You can see this in gas lamps. Fluorescent lamps, neon signs and sodium-vapor lamps are common examples of this kind of electric lighting, which passes an electric current through a gas to make the gas emit light. The colors of gas lamps vary widely depending on the gas and construction of the lamp. A sodium vapor light energizes sodium atoms to generate photons. Sodium has 11 electrons with many levels of orbitals. The wavelength for sodium corresponds to yellow light and if you run its light through a prism, you don't see a rainbow - you see a pair of yellow lines.

Sunlight is Born
Gamma rays are the highest-energy form of light. Instead of escaping, gamma ray photons bounce off electrons, transferring some of their energy. That both keeps the Sun’s interior hot and after bouncing around the energy of the photons are reduced to an energy level of visible light. The immense gravity and size of the Sun can take, it is estimated, almost 200 thousand years for a photon to travel from the core to the surface and only 8 minutes to Earth.
But in our quest for the understanding of light, we gained an understanding of atomic theory that enabled us to harness the energy of the very stuff that powers the Sun itself.