Lucid Guideline For I.U.P.A.C. Nomenclature Of Organic Compounds: Part-3:
List of Topics Included (Continued from part-2)
(11) Nomenclature of alicyclic hydrocarbons
(11-1) Alicyclic hydrocarbons containing only one ring
(11-1/A) Cycloalkanes and Cycloalkenes
(11-1/B) Meaning of: alkyl, alkenyl and alkynyl branch
(11-1/C) Cycloalkanes with alkyl or alkenyl branch
(11-1/D) Cycloalkenes containing one or more alkyl branches
(11-2) Alicyclic hydrocarbons containing two rings
(11-2/A) Alicyclic hydrocarbons containing two rings joined by a single bond
(11-2/B) Alicyclic hydrocarbons containing two rings joined through a hydrocarbon group
(11-2/C) Alicyclic hydrocarbons containing two rings fused having one side in common (called bicyclo compounds) and
(11-2/D) Alicyclic hydrocarbons containing two rings fused with one carbon atom in common (called spiro compounds)
@ References
(11) Nomenclature of Alicyclic Hydrocarbons
Alicyclic hydrocarbons are such compounds in which carbon atoms are arranged in a cycle to form a ring like structure, and hydrogen atoms are joined with each carbon. They are also known as closed chain compounds.
Such compounds are produced when two terminal carbon atoms of an open chain compound join through a covalent bond after removal of hydrogen atom from each.
For example, when two hydrogen atoms from each of the terminal carbon atoms of propane are removed and then the terminal carbon atoms combine through a single covalent bond, an alicyclic compound known as, “Cyclopropane” is produced.
Thus even though they are saturated compounds, they have two hydrogen atoms less. Hence their general formula is (CnH2n) which is similar to those for alkenes which are unsaturated compounds.
For example, molecular formula of "cyclopropane" which is first member of homologous series of alicyclic hydrocarbons is C3H6. This is similar to that of propene which is an unsaturated compound called alkene.
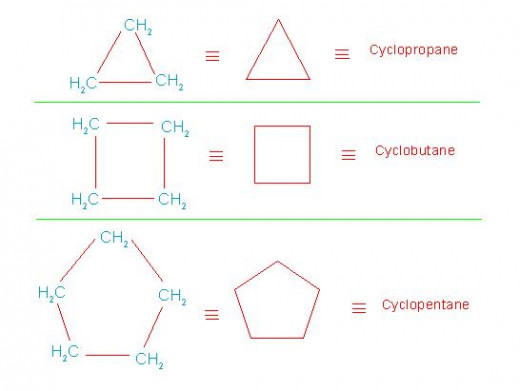
[Note: Due to cyclic shape of these compounds and tetrahedral shape of each carbon, it is difficult to draw their true structures. Hence their structures are represented by suitable geometrical symbols like: regular triangle, regular pentagon etc. These symbols actually denote their bond line structures].
Alicyclic hydrocarbon compounds are divided into two major categories:
(a) Alicyclic hydrocarbons containing one ring and
(b) Alicyclic hydrocarbons containing two rings.
(11-1) Alicyclic hydrocarbons containing only one ring
These types of alicyclic hydrocarbons contain only one ring or cycle in which several carbon atoms are joined with each other.
This category is further divided into two sub-categories:
(a) Alicyclic hydrocarbon containing a ring only but no substituent or hydrocarbon branch and
(b) Alicyclic hydrocarbon containing a ring as well as some substituent or hydrocarbon branch.
These are discussed in detail in the following paragraph.
(11-1/A) Alicyclic Hydrocarbons containing a ring only but no substituent or hydrocarbon branch (also called, "cycloalkanes" and "cycloalkenes")
These compounds contain only a ring structure made up of several carbon atoms. Such compounds are of two types:
(a) cycloalkanes: These are the compounds in which all the carbon atoms as well as hydrogen atoms are joined through a single bond. They are saturated alicyclic hydrocarbon compounds.
(b) cycloalkenes: These are the compounds in which there is at least one double bond between two carbon atoms. They are unsaturated alicyclic hydrocarbon compounds.
As, a minimum of three carbon atoms are required to form a cyclic structure, the first member of their homologous series contains three carbon atoms.
Homologous series of cycloalkanes and that of cycloalkenes are shown in following pictures.
Homologous series of cycloalkanes
Homologous series of cycloalkenes
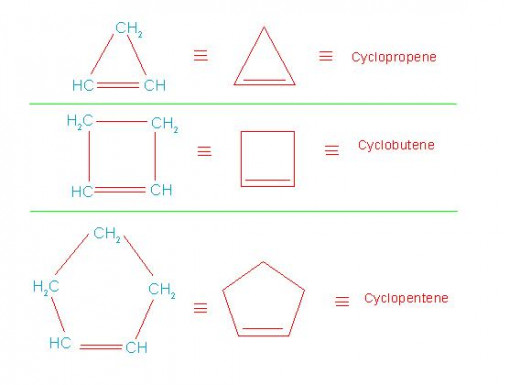
(11-1/B) Meaning of alkyl, alkenyl and alkynyl branch
What is called a branch?
In the given organic compound, the longest and continuous chain of carbon which is selected to assign root name, is called principle chain. However, any other smaller hydrocarbon groups which are attached to the principle chain are known as branches. Such branches are of three types:
(a) Alkyl branch
(b) Alkenyl branch and
(c) Alkynyl branch.
The detailed information about them is given below.
(a) What is called, "alkyl branch"?
The branches like: methyl (having formula: -CH3), ethyl (having formula: -CH2-H3) or propyl (having formula: -CH2-CH2-H3) etc. are called alkyl branches. Such type of branch is derived by removing one hydrogen atom from alkane. They contain only single bonds between two carbon atoms.
For example, when one hydrogen atom is removed from methane, an alkyl branch called "methyl branch" is produced. Likewise removal of one hydrogen atom from ethane gives "ethyl branch".
(b) What is called, "alkeneyl branch"?
The branches like: vinyl (having formula: -CH=CH2) and allyl (having formula: -CH2-CH=CH2) are called alkeneyl branches. Such type of branch is derived by removing one hydrogen atom from alkene. They contain at least one carbon-carbon double bond.
For example, when one hydrogen atom is removed from ethene, an alkenyl branch called "vinyl branch" (also known as "ethenyl branch") is produced. Likewise removal of one hydrogen atom from propene gives "allyl branch".
(c) What is called, "alkynyl branch"?
The branches like: ethynyl and propynyl are called alkynyl branches. Such type of branch is derived by removing one hydrogen atom from alkyne. They contain at least one carbon-carbon triple bond.
For example, when one hydrogen atom is removed from ethyne, an alkynyl branch called "ethynyl branch" is produced.
They are rare and uncommon.
Hence examples of compounds containing such branches are only few.
(11-1/C) Cycloalkanes containing alkyl or alkenyl branch
(A) Naming cycloalkanes containing only one alkyl branch:
The I. U. P. A. C. names of alicyclic compounds containing branches are determined on the basis of number of carbon atoms present in the ring and that present in the alkyl group.
This can be explained by following three rules:
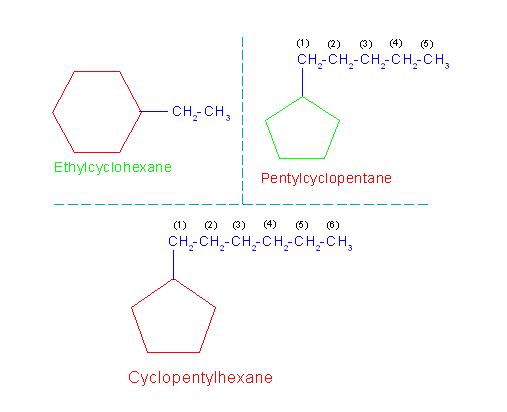
(1) If the number of carbon atoms is greater in ring than that in branch, then the ring is regarded as main compound while branch is regarded as substituent. In such a case the general name of compound is given as, "Alkylcycloalkane".
For example, consider naming of compound: ethylcyclohexane. Here, number of carbon atom is six in the ring but is only two in alkyl group. Hence compound is regarded as ethyl derivative of cyclohexane and named as, "ethylcyclohexane".
(2) However, if number of carbon atoms is greater in branch than that in ring, then the alkyl branch is regarded as main compound while ring as substituent. In such a case the general name of compound is given as, "Cycloalkylalkane".
For example, consider naming of compound: cyclopentylhexane. Here, number of carbon atom is five in the ring but is six in alkyl group. Hence compound is regarded as cyclopentyl derivative of hexane and named as, "cyclopentylhexane".
(3) If number of carbon atoms is same in branch and that in ring, then the ring is considered as main compound while alkyl branch is regarded as substituent. In such a case the general name of the compound is given as, "Cycloalkylalkane".
For example, consider naming of compound: pentylcyclopentane. Here, ring as well as branch contains five carbon atoms each. Hence compound is regarded as pentyl derivative of cyclopentane and named as, "pentylcyclopentane".
The following picture number: 11-1/C/1 will help to understand the naming of above discussed compounds.
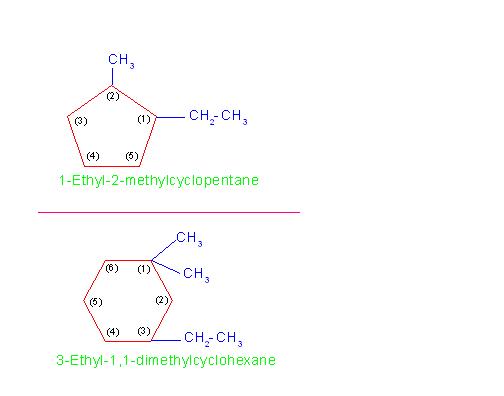
(B) Naming cycloalkanes containing two or more alkyl branches:
Nomenclature of such compounds is governed by following rule:
"When two or more hydrocarbon branches are attached to the ring, then their relative positions are mentioned by suitable numbers".
Here also, both alphabetical order rule and lowest set of locant rule are obeyed. Further, the more substituted carbon atom of the ring should bear lower number.
For example naming of compounds: 1-Ethyl-2-methylcyclopentane and 3-Ethyl-1,1-dimethylcyclohexane.
See the picture number: 11-1/C/2.
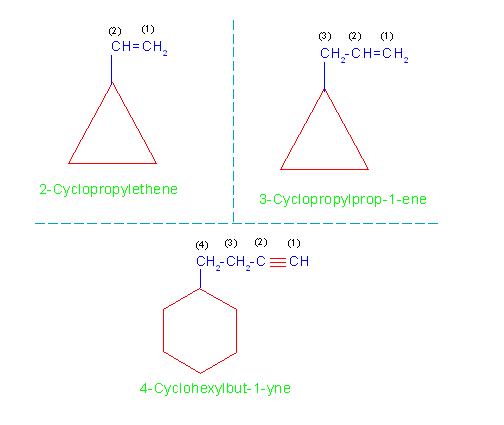
(C) Naming cycloalkanes containing alkenyl or alkynyl branch:
However, if an alkenyl or an alkynyl group (means a hydrocarbon group containing double or triple bond) is attached to the ring, then irrespective of number of carbon atoms, the alicyclic ring is always considered as a substituent while the hydrocarbon group containing a multiple bond is always considered as a principle compound.
For example naming of compounds:
(a) 2-Cyclopropylethene (but not 1-Cyclopropylethene)
(b) 3-Cyclopropylprop-1-ene and
(c) 4-cyclohexylbut-1-yne
See the picture number: 11-1/C/3.
Picture number: 11-1/C/1
Picture number 11-1/C/2
Picture number: 11-1/C/3
(11-1/D) Cycloalkenes containing one or more alkyl branches
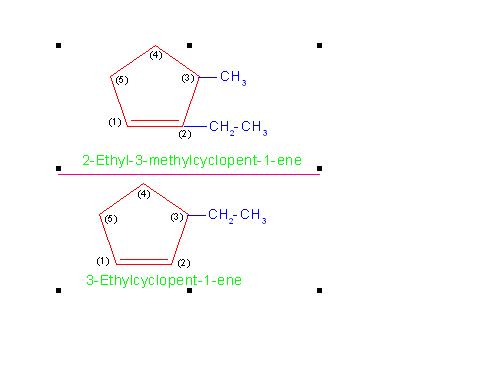
If ring contains a double bond but smaller hydrocarbon group attached to ring does not contain any multiple bond, then numbering is done in such a way that the multiple bond of the ring gets lower number.
For example:
(a) 2-Ethyl-3-methylcyclopent-1-ene and
(b) 3-Ethylcyclopent-1-ene
See the picture number: 11-1/D
Picture number: 11-1/D
(11-2) Alicyclic Hydrocarbons containing two rings
The alicyclic compounds containing two rings have a variety of possible structures. Depending upon the nature of combination of two rings, they are further divided into following sub categories:
(11-2/A) Alicyclic Hydrocarbons containing two rings joined with a single bond
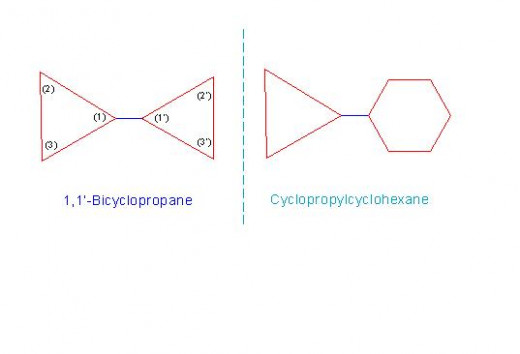
The characteristic of such compounds is that, the carbon atom of one ring is joined with carbon atom of another ring through a single covalent bond. In such cases, numbering of carbon atoms of one ring is given as: 1, 2, 3....; while that of another ring is given as: 1', 2', 3'...etc.
In case, both the rings are similar, the compound is named as, "z,z'-Bicycloalkane". Here, the numbers z and z' denote the number of carbon atoms of adjacent rings through which they are joined.
For example a compound called, "1,1'-Bicyclopropane".
However if both the rings are different, then the smaller ring (with lesser number of carbon atoms) is considered as a substituent while the bigger ring i considered as a principle compound. General names of such compounds are: "cycloalkylcycloalkane".
For example a compound called, "Cyclopropylcyclohexane".
See the figure: 11-2/A
Picture number: 11-2/A
(11-2/B) Alicyclic Hydrocarbons containing two rings joined through a hydrocarbon group
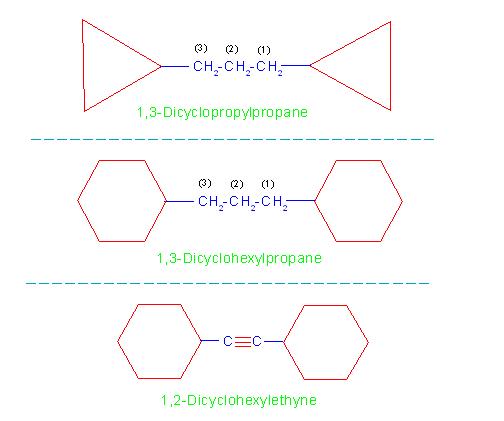
When two rings are joined by a hydrocarbon group (which may be: an alkyl group, alkenyl group or alkynyl group), the compound is considered as a cycloalkane derivative of that group.
Here, number of carbon atoms in the ring or in the hydrocarbon group is not considered.
The general name of such compounds is: "x,y-Dicycloalkylalkane", where x and y are numbers of carbon atoms of hydrocarbon group on which the two rings are attached.
For example naming compounds:
(a) 1,3-Dicyclopropylpropane
(b) 1,3-Dicyclohexylpropane and
(c) 1,2-Dicyclohexylethyne.
See picture number: 11-2/B
Picture number: 11-2/B
(11-2/C) Alicyclic Hydrocarbons containing two fused rings having one side common (Bicyclo Compounds)
The naming of this special class of compounds requires a special knowledge as explained through following points.
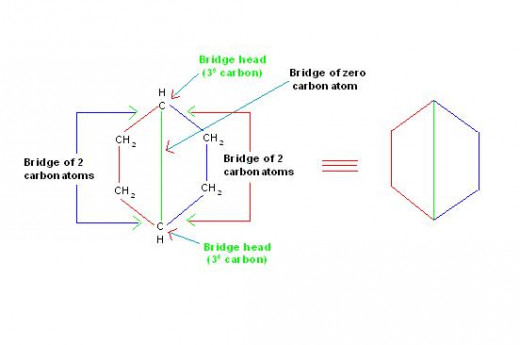
(1) These compounds contain; 2 rings, attached by 2 tertiary (30) carbon atoms to give 3 discrete bridges. (The tertiary carbon is such carbon atom which is joined with other three carbon atoms. It is denoted by symbol, "30-carbon".
[Note:
(a) The two 30 carbon atoms are known as, “bridge heads” and
(b) A covalent bond or an alkyl chain joining the two "bridge heads" is known as, “bridge”.]
(2) These compounds are represented by general name: “Bicyclo[x,y,z]alkane”.
[Note:
(a) x, y & z are numbers of carbon atoms (excluding the tertiary carbon atom) contained in various bridges and arranged in descending order.
(b) Alkane is the name in homologous series of alkane, corresponding to total number of carbon atoms present in both the rings.]
(3) The numbering of various carbon atoms is done on the basis of following three rules :
(a) First of all, one of the tertiary carbon atoms is selected and assigned number-1.
(b) Then proceeding numbers are given in such a way that longest bridge comes first while the smallest bridge comes at last.
(c) In case a functional group is present on one of the bridges, then numbering of that particular bridge should be done such that the functional group carries the lowest possible number.
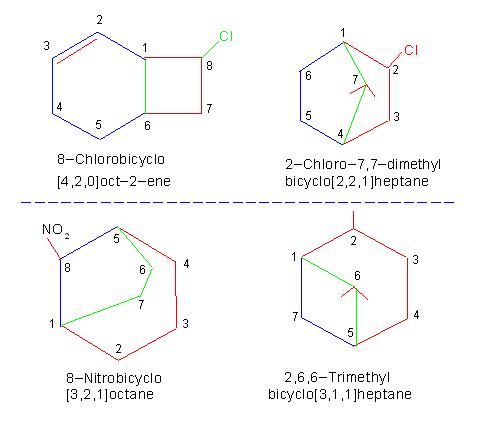
The picture number: 11-2/C/1 given below explains the structure of compound: "Bicyclo[2,2,0]hexane". The picture also illustrates meaning of "Bridge" and that of "Bridge Head".
The picture number: 11-2/C/2 given below illustrates I. U. P. A. C. names and structures of some bicyclo compounds.
Picture number: 11-2/C/1
Picture number: 11-2/C/2
(11-2/D) Alicyclic Hydrocarbons containing two fused rings having one carbon atom in common (Spiro Compounds)
Such compounds contain 2 rings, attached by a common quaternary carbon atom. Quaternary carbon means carbon atom which is joined with four other carbon atoms. It is denoted by symbol: "40-carbon".
Nomenclature of such compounds is done considering following guidelines.
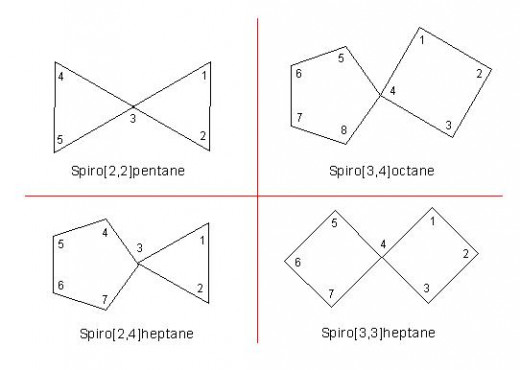
(1) They are represented by common name: “spiro[x,y]alkane”.
Here, "x" and "y" which are included in square bracket are the numbers of carbon atoms in different rings. These numbers should not include quaternary carbon atom and they should be written in ascending order.
(2) The word "alkane" used in their names is the name of corresponding alkane which contains similar number of total carbon atoms as contained by spiro compound.
(3) For the purpose of numbering, the carbon atom which is next to quaternary carbon is assigned number 1. Then numbering proceeds along both the rings in the order of smaller ring to larger ring.
[Note: Here, quaternary carbon as well as its former and latter carbon atoms, all three should remain in straight line].
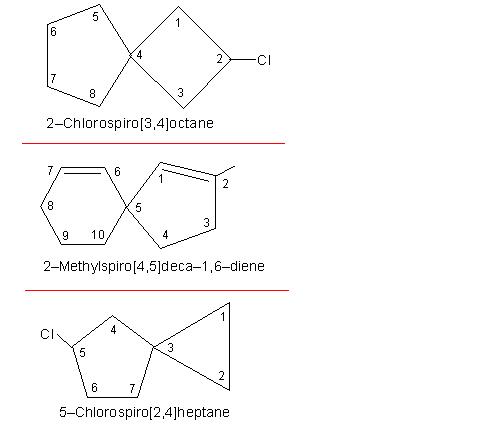
Illustrations of both simple spiro compounds and complex spiro compounds are given in the following picture number: 11-2/D/1.
Picture number: 11-2/D/1
@ References
(1) Organic chemistry by: Robert Thornton Morrison and robert Neilson Boyd, Seventh Edition, Published by, "Dorling Kindersley(India) Pvt. Ltd., licensees of Pearson Education in South Asia
(2) Oxford Dictionary Of Chemistry, published by Oxford University Press Inc., New York
(3) I. I. T. Chemistry, by Dr. O.P. Agarwal, 135th edition, Jai Prakash Nath Publications, Meerut, India
(4) Pradeep's New Course Chemistry, Class XI, Vol. II, 27th edition, Pradeep Publication, Jalandhar, India
(5) Pradeep's New Course Chemistry, Class XII, Vol. II, 27th edition, Pradeep Publication, Jalandhar, India
(6) Fundamentals Of Chemistry, Class 11, by J. D. Lee, Solomons & Fryhle, Published by: Wiley India Pvt. Ltd., 4435-35/7, Ansari Road, Daryaganj, New Delhi-110002
(7) Modern's abc of Chemistry, For Class XI, Part-II, by Dr. S. P. Jauhar, Published by: Modern Publishers, MBD House, Railway Road, Jalandhar City, India
(8) Modern's abc Of Chemistry, For Class XII, Part-II, by Dr. S. P. Jauhar, Published by: Modern Publishers, MBD House, Railway Road, Jalandhar City, India
(9) Organic Chemistry, by Bhupinder Mehta & Manju Mehta, Published by: Prentice-Hall Of India Private Limited, M-97, Connaught Circus, New Delhi, -110001, India
(10) Nootan ISC Chemistry, Class XI & XII, by Dr. H. C. Srivastava, Published by: Nageen Prakashan (Pvt.) Ltd., 310, Western Kutchery Road, Meerut-250001, U.P., India
Previous:
- Lucid Guideline For I.U.P.A.C. Nomenclature Of Organic Compounds: Part-2:
Do you know alkenes are more reactive than alkynes? So, while naming double bond should carry lower number? Do you know principal chain should contain double or triple bond irrespective of length?
Next:
- Lucid Guideline For I.U.P.A.C. Nomenclature Of Organic Compounds: Part-4:
Do you know there are two types of aromatic hydrocarbons? Benzenoids contain at least one benzene ring while non-benzenoids contain no benzene ring at all.