Lucid Guideline For I.U.P.A.C. Nomenclature Of Organic Compounds: Part-5:
List of Topics Included (continued from part-4)
(13/C) Nomenclature of organic compounds containing halogen (-X, X= F, Cl, Br or I) functional group
(13/D) Nomenclature of organic compounds containing amino (-NH2) functional group
(13/E) Nomenclature of organic compounds containing aldehyde (-CHO) functional group
(13/F) Nomenclature of organic compounds containing ketone (>C=O) functional group
(13/G) Nomenclature of organic compounds containing carboxyl (-COOH) functional group
(13/H) Nomenclature of organic compounds containing ester (-COO-) functional group
(13/I) Nomenclature of organic compounds containing cyano (-CN) functional group
(13/J) Nomenclature of organic compounds containing amide (-CONH2) functional group
(13/K) Nomenclature of organic compounds containing nitro (-NO2) functional group
(14) Nomenclature of organic compounds containing two functional groups
(14/A) Nomenclature of organic compounds containing two similar functional groups
(14/B) Nomenclature of organic compounds containing two dissimilar functional groups
(15) Nomenclature of organic compounds containing three similar functional groups
@ References
(13/C) Nomenclature of organic compounds containing halogen functional group
Halogen group is named as, "halo" group and is denoted by symbol -X.
For example: -F = fluoro group, -Cl = chloro group, -Br = bromo group and -I = iodo group.
In common system of naming, these compounds are known as alkyl halide or aryl halide.
But in I. U. P. A. C. system of naming, all halo groups are used as prefixes. Following examples will help to understand this.
(a) CH3-F is called, “Fluoromethane” (its common name is methyl fluoride),
(b) CH3-CH2-Cl is called, “Chloroethane” (its common name is ethyl chloride),
(c) CH3-CH2-CH2-Br is called, “Bromopropane” (its common name is propyl bromide) and
(d) CH3-CH(I)-CH2-CH2-CH3 is called, “2-Iodopentane”.
(13/D) Nomenclature of organic compounds containing amino functional group
Amino group is called amine group and is denoted by symbol -NH2.
In common system of naming, these compound are known as alkyl amine or aryl amine.
However, in I. U. P. A. C. system of naming suffix, “amine” is added after root name. Here the terminal "e" in the name of alkane is dropped. This will be clear from following examples.
(a) CH3-NH2 is called, “Methanamine” (its common name is methyl amine),
(b) CH3-CH2-NH2 is called, “Ethanamine” (its common name is ethyl amine),
(c) CH3-CH2-CH2-NH2 is called, “Propanamine” (its common name is propyl amine),
(d) CH3-CH(NH2)-CH3 is called, “Propan-2-amine” (its common name is isopropyl amine) and
(e) CH3-CH(NH2)-CH2-CH3 is called, “Butan-2-amine” (its common name is sec-butyl amine).
(13/E) Nomenclature of organic compounds containing aldehyde functional group
In I. U. P. A. C. system of naming, compounds containing aldehyde group (-CHO) are named using suffix, “al”. This means to derive their names, suffix “al” is added after root name. Here the terminal "e" of the root name is dropped. This will be clear from following examples.
(a) H-CHO is called, “Methanal” (its common name is formaldehyde),
(b) CH3-CHO is called, “Ethanal” (its common name is acetaldehyde),
(c) CH3-CH(CH3)-CHO is called, “2-Methylpropanal” (its common name is isobutyraldehyde).
However, when aldehyde group is directly attached to some cyclic hydrocarbon ring, suffix “carbaldehyde” is used in place of suffix, “al”. Here, terminal “e” in the name of cyclic ring is retained. This will be clear from following examples.
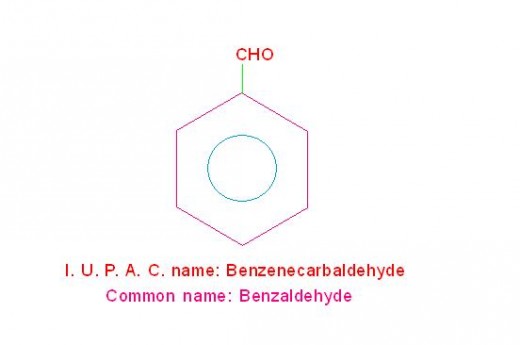
(d) C6H5-CHO is called, “Benzenecarbaldehyde" (its common name is benzaldehyde) and
(e) C6H11-CHO is called, “Cylohexanecarbaldehyde”.
See the picture given below.
Structure and I. U. P. A. C. name of benzaldehyde
(13/F) Nomenclature of organic compounds containing ketone functional group
Ketone group is called keto group and is denoted by symbol >C=O.
In I. U. P. A. C. system of naming, general name of compound containing keto group is, "alkanone”. Here, suffix “one” is added after removing terminal “e” of alkane name. This will be clear from following examples.
(a) CH3-CO-CH3 is called, “Propanone” (its common name is acetone or dimethyl ketone),
(b) CH3-CO-CH2-CH3 is called, “Butan-2-one” (its common name is ethyl methyl ketone),
(c) CH3-CH2-CO-CH2-CH3 is called, “Pentan-3-one” (its common name is diethyl ketone).
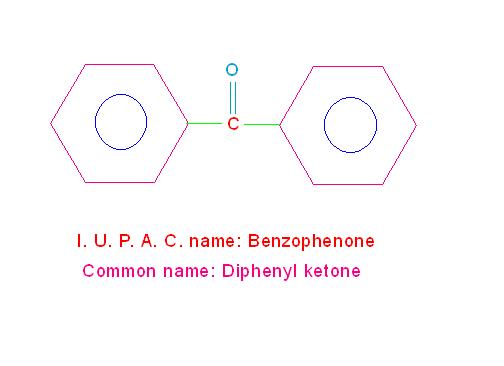
However, in case keto group is situated between two benzene rings compound is called, “Benzophenone”.For example:
(e) C6H5-CO-C6H5 is called, “Benzophenone” (its common name is, “Diphenyl ketone”).
See the picture given below.
Structure and IUPAC name of diphenyl ketone
(13/G) Nomenclature of organic compounds containing carboxyl functional group
Carboxyl group is denoted by symbol -COOH.
In I. U. P. A. C. system of naming, general name of compound containing carboxyl group is, “alkanoic acid”. Here, suffix “oic acid” is added after removing terminal “e” of alkane name. This will be clear from following examples.
(a) H-COOH is called, “Methanoic acid” (its common name is, formic acid),
(b) CH3-COOH is called, “Ethanoic acid” (its common name is, acetic acid),
(c) CH2=CH-COOH is called, “Prop-2-enoic acid” (its common name is, acrylic acid) and
(d) CH3-CH=CH-COOH is called, “But-2-enoic acid” (its common name is, crotonic acid).
However, in case carboxyl group is directly attached to the aromatic or alicyclic ring, suffix “carboxylic acid” is added after name of ring. This will be clear from following examples.
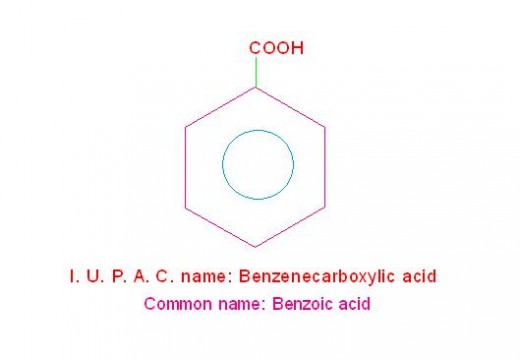
(e) C6H5-COOH is called, “Benzenecarboxylic acid” (its common name is, benzoic acid.
(See the picture given below).
(f) C6H11-COOH is called, “Cyclohexanecarboxylic acid”.
structure and I. U. P. A. C. name of benzoic acid
(13/H) Nomenclature of organic compounds containing ester functional group
Ester group is denoted by symbol -COO-.
Their general formula is, "RCOOR’, where R and R' are alkyl groups.
In I. U. P. A. C. system of naming, general name of compound containing ester group is, “alkyl alkanoate”. This is because these compounds are regarded as alkyl derivatives of carboxylic acids. This means when acidic “H” of carboxylic acid is substituted by some alkyl group, the resultant compound is known as ester compound.
To assign correct I. U. P. A. C. name to the given ester compound following guide line is helpful.
(a) The given ester compound is first divided in two parts such that ester group (-COO-) remains attached with the bigger alkyl group.
(b) The smaller alkyl group is named as its corresponding alkyl name, while the bigger alkyl group containing ester group is named as corresponding alkanoate.
This will be clear from following examples:
(a) H-COO-CH3 is called, “Methyl methanoate” (its common name is methyl formate),
(b) CH3-COO-CH3 is called, “Methyl ethanoate” (its common name is methyl acetate),
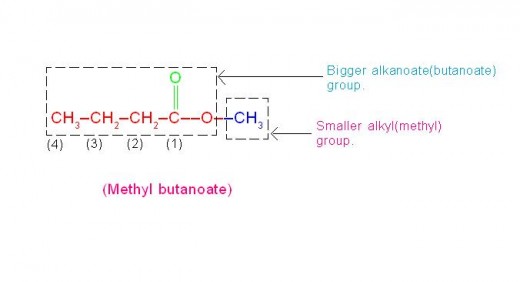
(c) CH3-CH2-CH2-COO-CH3 is called, “Methyl butanoate” (which has flavor of Apple),
(d) CH3-CH2-CH2-COO-CH2-CH2-CH2-CH3 is called, “Butyl butanoate” (which has flavor of Pineapple).
Please see the picture given below.
Can you assign correct I. U. P. A. C. names to following ester compounds?
view quiz statistics(13/I) Nomenclature of organic compounds containing cyano functional group
In common system of nomenclature, cyano group is also called cyanide group. It is denoted by symbol -CN.
However, In I. U. P. A. C. system of naming, cyano group is called “Nitrile group”. General name of compound containing cyano group is, “alkanenitrile”.
The terminal “N” of cyano group is named as, “nitrile”.
To assign the correct name to such compound, alkane name corresponding to continuous carbon chain is determined first and then suffix nitrile is added after it. This will be clear from following examples.
(a) CH3-CN is called, “Ethanenitrile”. Its common name is, “methyl cyanide” or “cyano methane”.
(b) CH3-CH2-CN is called, “Propanenitrile”. Its common name is, “ethyl cyanide” or “cyano ethane”.
However, in case -CN group is directly attached to aromatic or alicyclic ring, the suffix “carbonitrile” is used in place of nitrile. This will be clear from following examples.
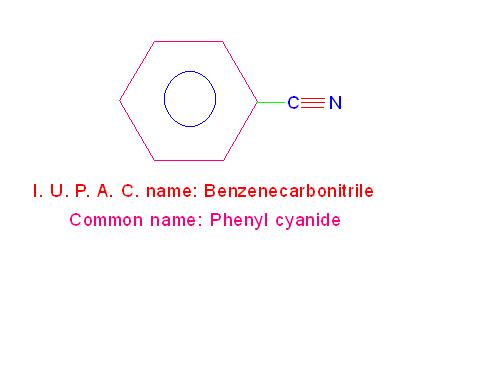
(c) C6H5-CN is called, “Benzenecarbonitrile”. Its common name is, “phenyl cyanide”.
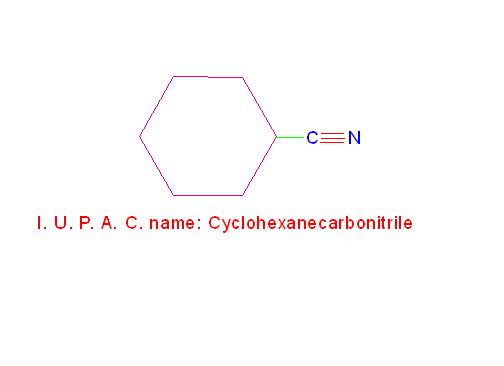
(d) C6H11-CN is called, “Cyclohexanecarbonitrile”.
See the picture given below.
Structures and I. U. P. A. C. names of phenyl cyanide and cyclohexyl cyanide
(13/J) Nomenclature of organic compounds containing amide functional group
Amide group is denoted by symbol -CONH2.
In I. U. P. A. C. system of naming, general name of compound containing amide group is, “Alkanamide”. Here, alkane name corresponding to continuous carbon chain is determined first. Then suffix “amide” is added after removing “e” from alkane name. This will be clear from following examples.
(a) CH3-CONH2 is called, “Ethanamide”. Its common name is acetamide.
(b) CH3-CH2-CH2-CONH2 is called, “Butanamide”.
(13/K) Nomenclature of organic compounds containing nitro functional group
Nitro group is denoted by symbol -NO2.
In I. U. P. A. C. system of naming, general name of compound containing nitro group is, “Nitroalkane”. Here, alkane name corresponding to continuous carbon chain is determined first. Then prefix nitro is added before alkane name. This will be clear from following examples.
(a) CH3-NO2 is called, “Nitromethane”,
(b) CH3-CH2-CH2-NO2 is called, “Nitropropane”.
(14) Nomenclature of organic compounds containing two functional groups
Compounds containing two functional groups are known as, "bifunctional compounds".
They are further divided into two categories:
(a) compounds containing two similar functional groups; and
(b) Compounds containing two dissimilar functional groups.
Methods of naming such compounds are discussed in the following sections.
(14/A) Nomenclature of organic compounds containing two similar functional groups
In case a given compound contains two similar groups, then naming is done on the basis of following guidelines.
(1) First of all the root name of compound is determined based on number of carbon atoms in continuous carbon chain.
(2) Then prefix or suffix used for the functional group present is determined.
(3) Then prefix "di" is added before the name of prefix or suffix of the functional group. For example: dichloro, dibromo, dinitro, diol, dione etc.
(4) To show the location of both the groups, numerical prefixes like 1,2 or 2,4 etc. are added before the name of functional group. This will be clear from following examples.
(a) CHCl3 is called, “Trichloromethane”. Its common name is, “chloroform”.
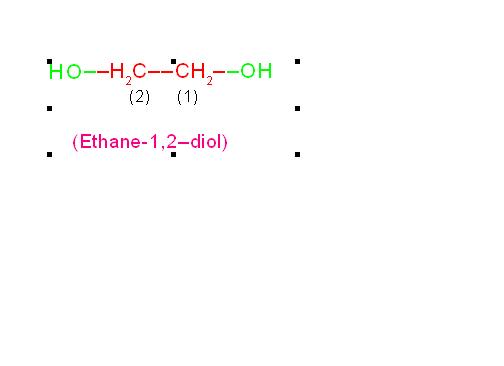
(b) HO-CH2-CH2-OH is called, “Ethane-1,2-diol”. Its common name is, “ethylene glycol”.
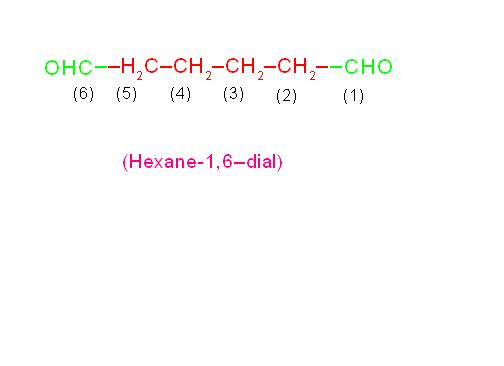
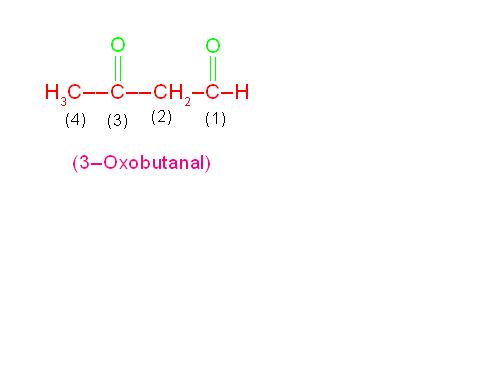
(c) OHC-CH2-CH2-CH2-CH2-CHO is called, “Hexane-1,6-dial” and
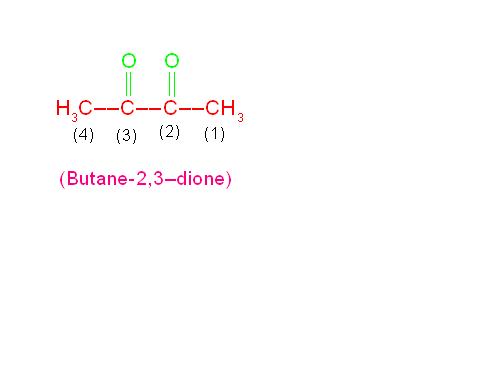
(d) CH3-CO-CO-CH3 is called, “Butane-2,3-dione”.
See the following pictures.
Structures and nomenclature of compounds containing two similar functional groups
(14/B) Nomenclature of organic compounds containing two dissimilar functional Groups
In case a given compound contains two dissimilar groups, then prefix or suffix of both the groups are added to the root name of compound.
However, the important point is that both the functional groups are not given similar priority. A functional group which is more reactive is given higher priority. This means the numbering to the continuous carbon chain should be done in such a way that group with higher priority gets lower number. The priority order (which is also known as reactivity order) of various functional groups is as follows:
-COOH > -SO3H > -COOR > COCl > -CONH2 > -CN > -CHO > C=O > -OH > NH2 > C=C etc.
Here, the more reactive group is considered as main group hence is given lower number and its suffix is added to the root name. But the less reactive group is considered as substituent hence given higher number and its prefix is added to the root name.
For example:
(a) H2N-CH2-CH2-CH2-COOH is called, “4-Aminobutanoic acid” because -COOH group is more reactive than -NH2 group.
(b) CH3-CH(Cl)-CH2-COOH is called, “3-Chlorobutanoic acid” because chloro group is less reactive than -COOH group.
(c) CH3-O-CH2-CH2-OH is called, “2-Methoxyethanol” because -OH group is more reactive than -OCH3 group.
See the following pictures.
Nomenclature of bifunctional compounds
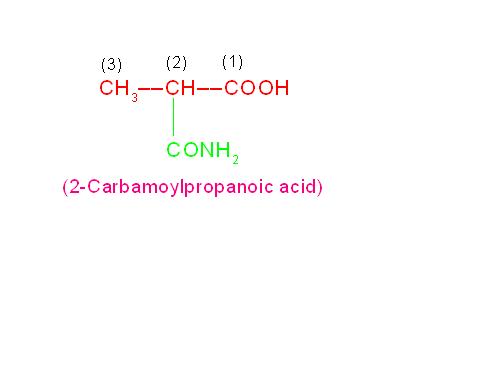
Can You Assign Correct I.U.P.A.C. Names to Following Bifunctional Compounds?
view quiz statistics(15) Nomenclature of organic compounds containing three similar functional groups
In case a given compound contains three similar groups, then without considering carbon atoms of functional groups, the compound is regarded as tri-derivative of parent hydrocarbon. For example:
(a) HOOC-CH2-CH(COOH)-CH2-COOH is called, “Propane-1,2,3-tricarboxylic acid” and
(b) NC-CH2-CH(CN)-CH2-CN is called, “Propane-1,2,3-tricarbonitrile”.
Is this all about I. U. P. A. C. nomenclature of organic compounds?
Not at all!
Assigning discrete I. U. P. A. C. names to each and every organic compound is a big task because there are numerous organic compounds.
Here, only some compounds containing common functional groups are included.
However, the compounds containing functional groups like: thiol (-SH), thial (-CH=S), imido (>NH), isonitrile (NC), nitrite (-O-N=O) etc. are not included as they are rare.
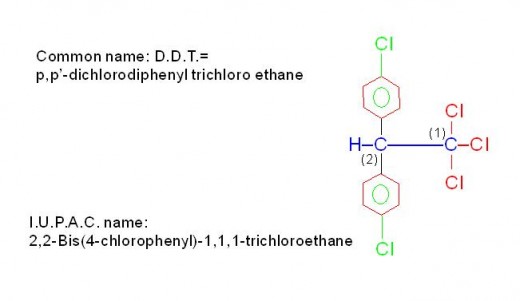
There are also some compounds like "D.D.T.", which contains more than three functional groups. Such compounds are known as, "Poly functional compounds". Naming of such compounds requires even deeper knowledge. See the picture given below.
However, these five hubs (part 1 to part 5) are generally adequate for naming most of common organic compounds.
Picture showing structure, common name and I. U. P. A. C. name of polyfunctional compound, "D. D. T."
@ References
(1) Organic Chemistry by: Robert Thornton Morrison and Robert Neilson Boyd, Seventh Edition, Published by, "Dorling Kindersley(India) Pvt. Ltd., licensees of Pearson Education in South Asia
(2) Oxford Dictionary Of Chemistry, published by Oxford University Press Inc., New York
(3) I. I. T. Chemistry, by Dr. O.P. Agarwal, 135th edition, Jai Prakash Nath Publications, Meerut, India
(4) Pradeep's New Course Chemistry, Class XI, Vol. II, 27th edition, Pradeep Publication, Jalandhar, India
(5) Pradeep's New Course Chemistry, Class XII, Vol. II, 27th edition, Pradeep Publication, Jalandhar, India
(6) Fundamentals Of Chemistry, Class 11, by J. D. Lee, Solomons & Fryhle, Published by: Wiley India Pvt. Ltd., 4435-35/7, Ansari Road, Daryaganj, New Delhi-110002
(7) Modern's abc of Chemistry, For Class XI, Part-II, by Dr. S. P. Jauhar, Published by: Modern Publishers, MBD House, Railway Road, Jalandhar City, India
(8) Modern's abc Of Chemistry, For Class XII, Part-II, by Dr. S. P. Jauhar, Published by: Modern Publishers, MBD House, Railway Road, Jalandhar City, India
(9) Organic Chemistry, by Bhupinder Mehta & Manju Mehta, Published by: Prentice-Hall Of India Private Limited, M-97, Connaught Circus, New Delhi, -110001, India
(10) Nootan ISC Chemistry, Class XI & XII, by Dr. H. C. Srivastava, Published by: Nageen Prakashan (Pvt.) Ltd., 310, Western Kutchery Road, Meerut-250001, U.P., India
Previous:
- Lucid Guideline For I.U.P.A.C. Nomenclature Of Organic Compounds: Part-4:
Do You Know There Are Two Types Of Aromatic Hydrocarbons? Benzenoids Contain At Least One Benzene Ring. But Non-Benzenoids Contain No Benzene Ring At All!
Next:
- How Cooling Is Produced By an Air-Conditioner Machine?
Does an air-conditioner work on the principles of Physics? Not at all! Though some mechanical devices are also installed, the fundamental functioning of an A/C is based on the principles of chemistry!