Trends And The Periodic Table
The Periodic Table Facts
The modern periodic table has the following characteristics:
- Each horizontal row is called a period.
- The elements (from left to right across the period) are arranged in order of their atomic numbers.
- Elements often show trends in properties across the period (this is called periodicity).
- Each vertical column is called a group.
- The groups go from 0-7 and then the transition metals in the middle of the periodic table.
- The elements of each group have similar properties.
- Elements in the same group have the same amount of electrons in their outer shells.
- Group 1 will have one electron in their outer shell, Group 1 will have 2, Group 3 will have 3 etc.
- Elements in the same group will also have the same types of orbitals.
- Across a period (left to right) elements change from metals to non-metals.

Periodicity
Ionisation Energies Across The Period:
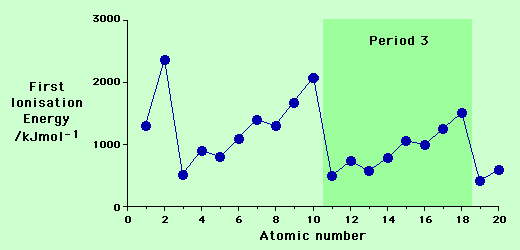
The first ionisation energy generally increases as you go across the period.
Remember that when speaking about ionisation energy you have to refer to
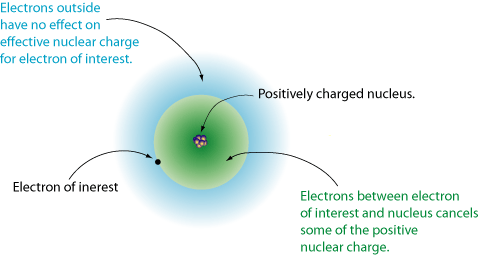
- Electron Shielding
- Nuclear Charge
- Distance From Nucleus
With this in mind, as you go across the period the amount of protons that an element has increases, this increases the nuclear charge of the element and thus the attraction between the outermost electrons and the positive nucleus.
As you go across the period the amount of electrons on the same shell increases, this means that the outer shell is drawn more towards the nucleus because there is a stronger attraction. This decreases the atomic radii and thus the distance from the nucleus.
The electron shielding wont change because the amount of inner shells is the same across the period.
All of this means that more energy is needed to remove the outermost electron and therefore as you go across a period (from left to right) the ionisation energy increases.

Trends Down A Group
First Ionisation Energy Down A Group:
The first ionisation energy decreases as you go down the group.
Again keeping in mind electron shielding, distance from the outermost electron and nuclear charge, the reasons for this are:
The number of electron shells increase as you go down the group, this means that the distance from the outermost electron and the electron shielding is increased and the attraction between the nucleus and the outermost electrons is weak.
The number of protons (nuclear charge) also increases, however this is far outweighed by the electron shielding and distance from outermost electron and therefore ionisation energy decreases as you go down the group.

Group 2 Trends
As you go down the group:
- The reactivity increases.
- The nuclear charge increases and the atomic radii increases,
- The electronegativity decreases.
- The melting point decreases down the group.
- There is no trend in boiling points.
Group 7 Trends
As you go down the group:
- The reactivity decreases.
- The atomic radii increases.
- The nuclear charge increases,
- Electronegativity decreases down the group.
- The melting and boiling points increases down the group.