Orbital Diagrams
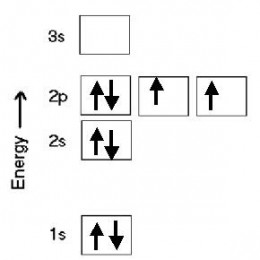
In an orbital diagram, electrons are represented by arrows. Boxes or blanks are used to represent orbitals. Upward-pointing arrows represent electrons with +1/2 spin; downward-pointing arrows represent electrons with -1/2 spin. Here's an example an orbital diagram. This is for an oxygen atom with an electron configuration of 1s2 2s2 2p4. There are eight arrows representing the eight electrons of an oxygen atom. Note that Pauli's principle must be followed: no more than 2 electrons per orbital; if there are two electrons in an orbital, one must be spin-up, the other spin-down.
Hund's Rule of Maximum Multiplicity
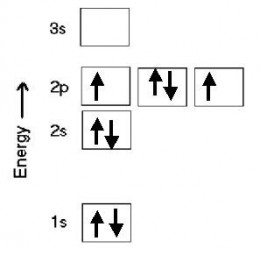
The orbital diagram shown for oxygen above shows just one of many (in this case, 15) allowed ways of distributing the electrons following the Aufbau principle. The one shown above represents one of the most stable, lowest energy distributions. To get this distribution, we fill the orbitals in the highest occupied subshell singly, with electrons of the same spin, before putting a second electron (of opposite spin) in any of the orbitals. When we do this, we are following what is known as Hund's Rule. Here's another example of an orbital diagram for oxygen that follows Hund's rule
To see orbital diagrams for the ground state of atoms, Click here
or watch this video:
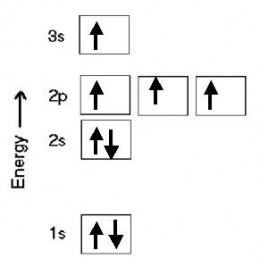
Excited state orbital diagram
Here's an orbital diagram that shows one of many ways of distributing electrons for an excited configuration of oxygen. The configuration in this case is 1s2 2s2 2p3 3s1
Paramagnetism: Magnetism of materials is due to unpaired electrons. Materials made of particles (atoms, molecules, or ions) that have one or more unpaired electrons are said to be paramagnetic. An oxygen atom, as shown above, has unpaired electrons. Oxygen atoms are like tiny magnets. They will be attracted to other magnets.
© 2015 Discover the World