Periodic Table of the Elements
Summary
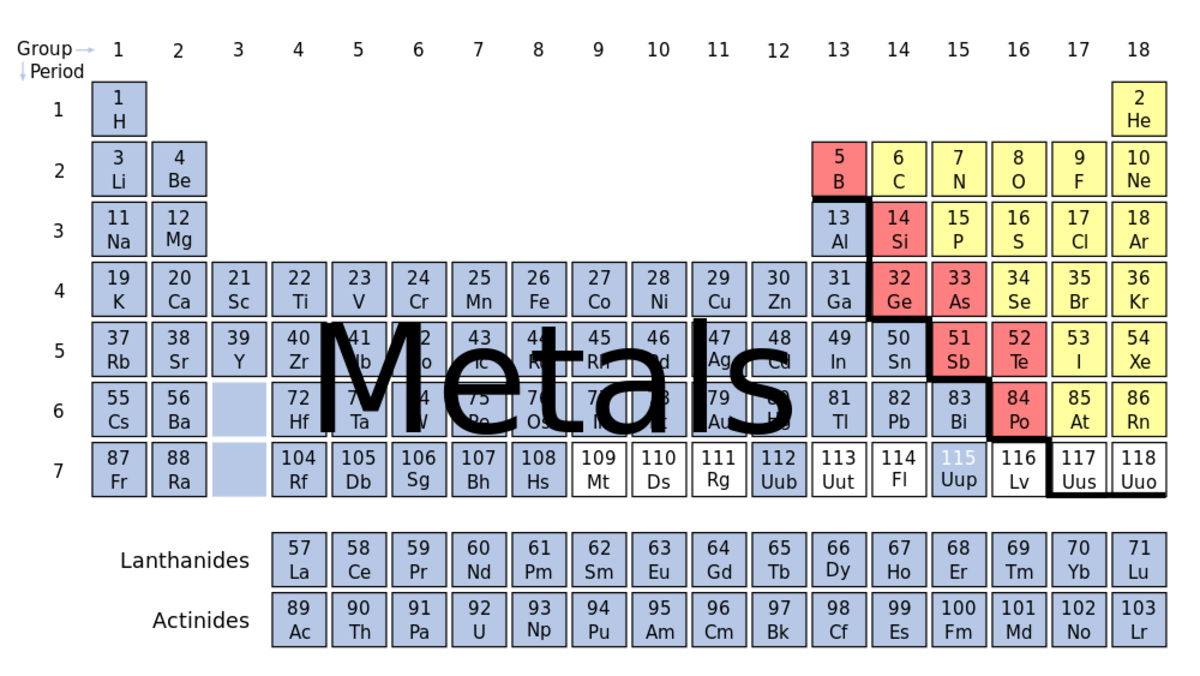
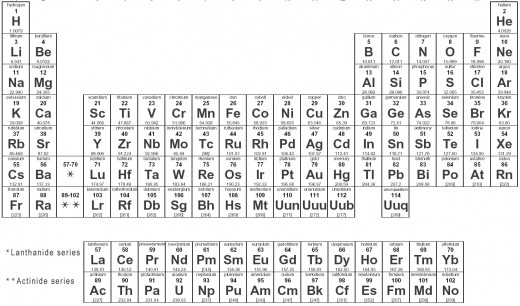
A table of all the known elements in the Universe displayed in a tabular method that groups common elements together. They are in order from lowest atomic number (upper left) to highest atomic number (lower right). Each element typically has in its box the name of the element, the element's symbol, the atomic number (the number of protons in the atom) and the atomic weight measured in amu (atomic mass units, 1 atomic mass unit = 1.660 538 782(83) × 10-27 kilograms. It is one twelfth of the mass of an isolated 12C atom).
The Periodic Table
Organization
The Rows are set in a specific manners in order that similar elements are in the same columns also known as groups. Every horizontal row, or period is the filling of an orbital with electrons from left to right on the table.
Groups
These are the vertical columns in the table. The elements in the groups have very similar properties because they have an equal number of electrons in their outermost orbital. Examples include: Group 1: alkali metals, Group 2: alkaline earth metals, Group 17: halogens, and Group 18: noble gases.
Periods
These are the horizontal rows in the table. Some of the rows show noticeable similarities like the d-block elements also known as the "transition metals." Also, f-block elements have similarities in their rows, such as the lanthanides and actinides.
Examples
Alkali Metals
The alkali metals (group 1) are never found in elemental form in nature, and are highly reactive. They have one electron in the outermost shell, so they are often found in nature as a positive cation. Alkali metals are often reactive with halogens to form ionic salts, and also are reactive with water forming strongly basic hydroxides.
Alkaline Earth Metals
The alkaline earth metals (group 2) are also quite reactive. They have two electrons in the outermost orbital, so are often stable in the 2+ ionization form. Alkaline earth metals form oxides, and often salts with halogens and group 16 elements. They too form hydroxides, but are weaker bases than the alkali metal hydroxides.
Noble gases
The noble gases are in Group 18. The notable characteristic of these elements is that they have full valence electron shells. Therefore, they are very unreactive because they do not need to react with another element to obtain a full shell.
Halogens
The halogens are in Group 17. The notable characteristic of these elements is that they are missing just one electron needed to fill the valence (outermost) electron shell. These in turn are very electronegative meaning that they tend to acquire electrons. These are very reactive elements. Also, halogens tend to react with hydrogen to form acids of the form HX.
Transition metals
Transition metals are Groups 3 to 12. There are noticeable trends across periods, not just down groups. These elements are not dramatic in difference across adjacent groups. An interesting property of the transition elements as a group is their partly filled d subshells.
Lanthanides and actinides
Lanthanides and actinides consist of f-block elements. Lanthanides are elements 57-71, and actinides are elements 89-103. These elements have very similar properties and are often difficult to separate when they are in a mixture. Uranium is one of these elements, which explains why it is difficult to obtain pure Uranium.
Metalloids
Metalloids are known for having properties of metals and nonmetals. The metalloids are Boron, Aluminum, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium. They often form amphoteric oxides, and are usually semiconductors. The "staircase" of metalloids which is diagonal from Boron to Polonium separates metals from nonmetals. The upper-right elements display more nonmetal behavior, while the more lower-left elements display more metallic behavior.
History
Dmitri Mendeleev is considered by many to be the creator of the periodic table, but there were many previous attempts at grouping the 63 known elements of his time. Mendeleev's creation was the best because it related elements not only vertically and horizontally, but also diagonally in some cases. Lord Rayleigh is credited with the discovery of the noble gas argon around 1895.
References
© 2015 Discover the World