Properties of Water

Water; a Curious Chemical Molecule
Water is much more than the H2O that we learned was the chemical make up of hydrogen and oxygen. Water in liquid form is essential for life as we know it. Although it has three main states within the atmosphere of the Earth, mainly solid, liquid and as a gas; under closer examination water has a multitude of discrete phase states of existence. The Eskimos have eleven words to describe snow and ice and this has been confirmed through quantum mechanics concerning phase states of frozen water. Quantum mechanics also tells us that in the liquid state, water has several phase states, which has important implications for global warming, especially at the 80 degree F mark. Above the boiling point, which exists at various temperatures depending on ambient atmospheric pressure, water is a gas which exists in one basic state below the reduction temperature. Outside of an atmosphere, the freezing point and boiling point are the same temeperature. Without an atmopshere that exerts a pressure, water does not exist in a liquid state unless there is a lot of it under gravity and ice. Water is even a more curious substance in deep space as a result.
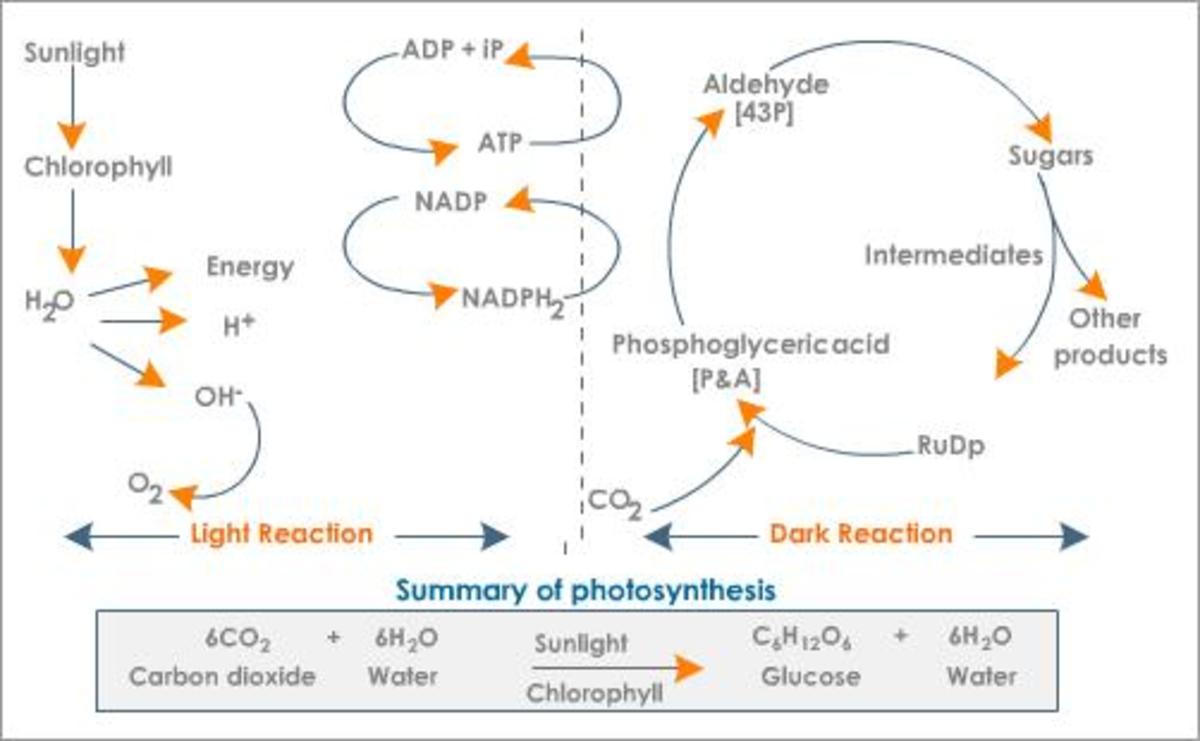
Water as a chemical has many properties when it interacts with other substances. Among them are heat absorption and retention, solvency, quantum density phase states, surface tension, cohesion, adhesion, capillary action and a dipole moment. Water is transparent at most electromagnetic wavelengths, which is essential for photosynthesis in underwater and land plants.
Heat absorption and retention of water makes it a heat sink but also a green house gas. Conversely, it can also be a cold sink. We can see this on a cloudy day were on summer days, the shade of clouds cools the earth below and on winter days, the earth is kept warmer. In the hydrologic cycle, a lot of solar heat is absorbed by the oceans, which evaporates the surface water and the heat remains until released to space or the cooling atmosphere. Ice crystals form as a result and it takes a lot of heat to change the state again. Water can evaporate at various temperatures thus the boiling point is fixed only to pressure. In deep space, it will evaporate at freezing temperature but near thermal vents at the bottom of the ocean, water can remain liquid even at 600 degrees Celsius.
As a solvent water is unequalled. This makes it perfect for all living processes and a lot more as well. The strong dipole moment of water will split molecules with a weaker dipole moment. These will then stick to the water until something stronger removes the atom or molecule from the water. In life, that process is photosynthesis that actually breaks down water in its primary stage in order to derive energy to run metabolism down the four stages of the process. Water not broken down assists in the process of delivering other molecules in the energy process of complex molecules that are important for growth and creation of energy storage in sugars.
The density of water is variable, but we usually refer to the specific density of liquid water at sea level as the bench mark for everything else. The specific density of water under the specialized condition of sea level on earth is given as .998 gm/cm^3 at one atmosphere for liquid and .917 cm/gm^3 for frozen water at one atmosphere. The maximum density of water is at 3.98 °C (39.16 °F) (1). Water becomes even less dense upon freezing, expanding about 9 percent. This causes an unusual phenomenon as ice floats upon water, and so water organisms can live inside a partly frozen pond because the water on the bottom has a temperature of around 4 °C (39 °F).
Surface tension results because of the dipole moment of water. Where water exists in abundance, water is attracted to water. Surface tension is great enough to allow some beetles to walk on the waters surface. Surface tension allows raindrops to be spherical and small organisms have difficulty coping in rain as a result of the surface tension of water. They can literally become trapped inside a raindrop and drown. Small organisms can cope with water internally as the dipole moment is regulated by the presence of other chemicals in the organism.
The dipole moment allows water to cohere to just about anything else. The electropositive and electronegative properties will allow water to cohere to both negatively and positively charged chemicals and substances. This is one reason why water will "wet" just about anything, except for oils and like substances. Due to cohesion, water sticks to itself and allows for things like capillary action.
Adhesion results when water loses a hydrogen atom and this is replaced by a hydrogen atom attached to something else. This process can occur as a result of electromagnetic radiation or from the intervention of photosynthesis. This is an extremely important property where life is concerned. Without this, many complex biological processes would not be able to occur.
Capillary action occurs due to the high dipole moment and self adhesion allows water to percolate in narrow spaces against the gravitational field. In simpler terms, this means water in the water table underground will rise in a wick like fashion to the surface of the ground through gaps in soil particles provided the soil is not too porous. In lowlands near rivers, crops grow easily due to this action. Once the water reaches the surface, heating causes evaporation and drying. As long as a water table is nearby, the water will continue to percolate up and keep soil moist. The same capillary action allows water to flow up trees in the trees vascular system. It is so efficient that trees can grow to hundreds of feet tall before the effect is cancelled by gravity and evaporation.
The dipole moment of water gives it the electrostatic charge needed to interact with other materials. The oxygen, which is highly reactive, has the greater electronegative charge than the hydrogen, which by comparison has a slight positive charge. Elements which are more electropositive than hydrogen such as lithium, sodium, calcium, potassium and cesium displace hydrogen from water, forming hydroxides. Being a flammable gas, the hydrogen given off is dangerous and the reaction of water with the more electropositive of these elements is violently explosive. Water thus can bond to a wide variety of elements and molecules and change too as a result. As a result of the dipole moment, water has a strong surface tension. Under the right conditions of temperature, this dipole moment will allow water to form a solid. But this solid state comes in a variety of forms due to quantum phase state changes from slush to hard ice. Ice can exist in a form where the alignment of water molecules will allow the solid state to float on the liquid state. This is the familiar form of ice we see in ice cubes and ice burgs. Under extreme pressure, a hot ice can form that will not float. This type of ice exists only where pressure is extreme enough to solidify it even at high heat. It is thought that on water worlds where there is no land surface above the water that are Earth mass or more, hot ice exists at the bottom where water rests on rock or metal. This ice forms under pressure and has a different structure than the ice we are familiar with. Experiments on Earth that involve water at extreme pressure demonstrate that this hot ice can exist. If we heat this ice considerably and suddenly release the pressure, a huge explosion will result.
On Earth, a combination of factors keeps most of the water in a liquid state. It thus is able under mainly solar influence to be involved in the hydrologic cycle to move through the gaseous, liquid and solid states.
References:
-
Kotz, J. C., Treichel, P., & Weaver, G. C. (2005). Chemistry & Chemical Reactivity. Thomson Brooks/Cole.